Ⅰ. Introduction
Peri-implantitis is defined as a plaque-associated pathology that causes inflammation of the peri-implant mucosa and progressive loss of supporting bone around dental implants.1,2 According to a retrospective analysis by Derks et al., peri-implantitis can develop at an early stage and is characterized by a non-linear and accelerating progression pattern.3 Management of peri-implantitis should include an initial nonsurgical step, during which bacterial deposits are to be removed through supra- and subgingival instrumentation. Following an evaluation of the healing response to nonsurgical treatment modalities and in case of residual signs of disease, surgical intervention is recommended. Studies have shown that nonsurgical therapy alone has limited efficacy in managing most cases of peri-implantitis. This limitation is likely due to the limited access to the implant surface, which hinders the effective cleaning of the contaminated and nonshedding surface. Therefore, surgical interventions are often required.4
The surgical treatment provides direct access to peri-implantitis lesion, enabling thorough debridement of infected tissue and effective decontamination of implant surfaces. It was effective in reducing inflammation and arresting disease progression.4,5 Various surgical approaches, including access flap surgery with or without adjunctive resective and/or augmentative procedures, have demonstrated improved outcomes in peri-implantitis treatment.5 Access flap surgery may lead to postoperative recession of the mucosal margin and consequent soft tissue dehiscences, potentially causing aesthetic problems. Augmentative approach aims to recreate ideal hard and soft-tissue conditions, in order to facilitate long-term maintenance and to preserve aesthetics.5
Additionally, augmentative approach seeks to regenerate bone defects and achieve re-osseointegration.5 Bone graft substitutes, barrier membranes, and biologically active materials such as enamel matrix derivatives (EMD) can be used for reconstruction of peri-implant tissues. Previous studies have indicated that narrower defect angles are more likely to result in greater radiographic bone fill during regenerative surgery.6Clinicians should consider augmentative procedures for treating peri-implantitis when implants have intrabony defects with a minimum depth of 3 mm and are contained by three or four walls, and also evaluate the presence of keratinized mucosa.5 However, case reports onreconstructive surgery for peri-implantitis remain limited.
The aim of this report is to introduce an augmentative approach for treating peri-implantitis, addressing three different types of bone morphology and bone defect types.
Ⅱ. Case Report
1. Case #1
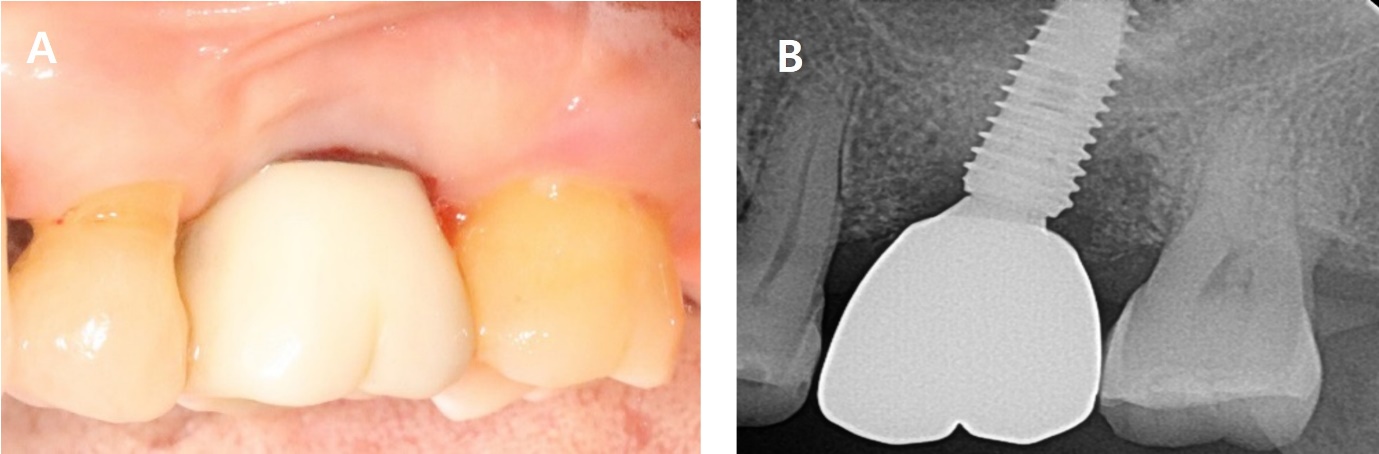
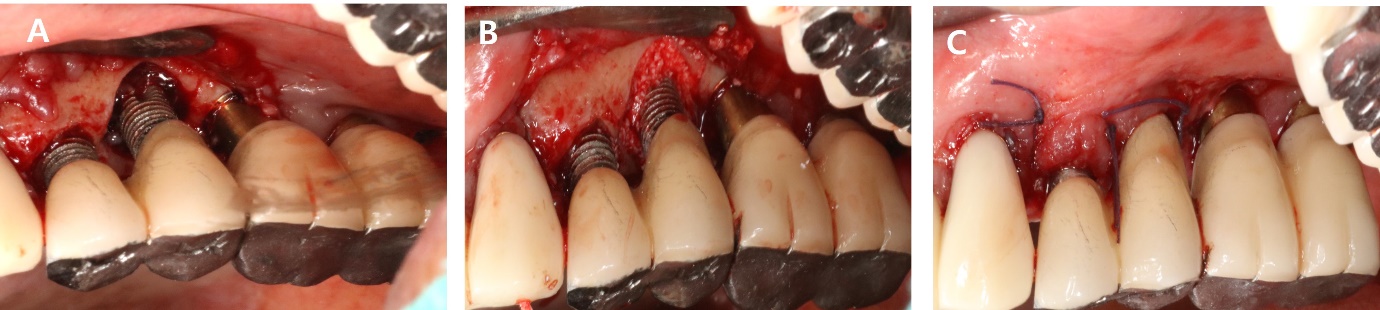
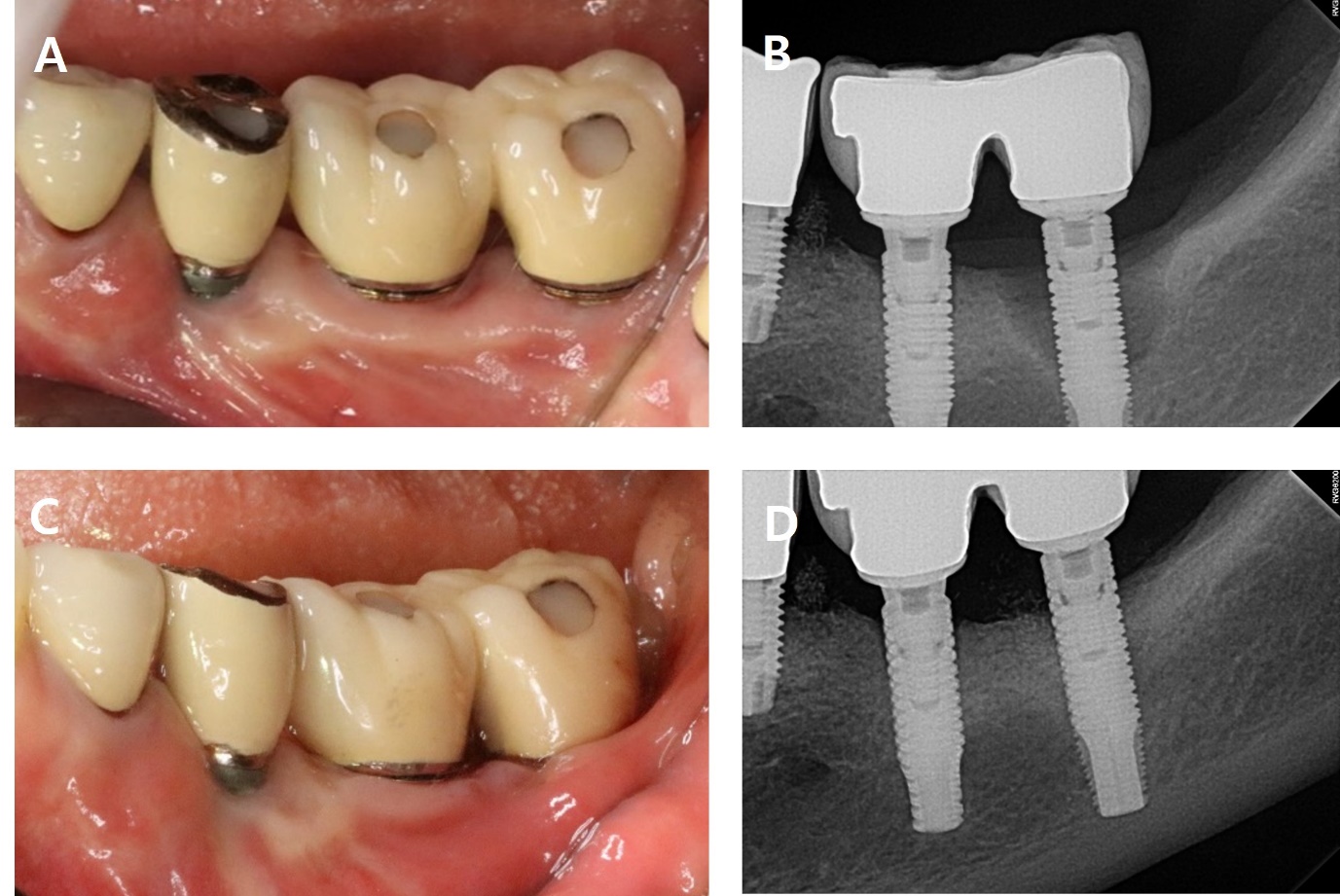
A 56-year-old woman presented with complaints of gingival bleeding around an implant placed at the left maxillary first molar (#26i). Clinical examination showed a 7 mm probing depth with suppuration. The radiographic findings revealed a moderate bone defect at the distal aspects of the implant, extending 5 mm from the crestal bone. Peri-implantitis was diagnosed on #26i. The patient underwent implantation 10 years ago and has been receiving regular maintenance. However, she reported pus discharge and bleeding for several years (Fig. 1).
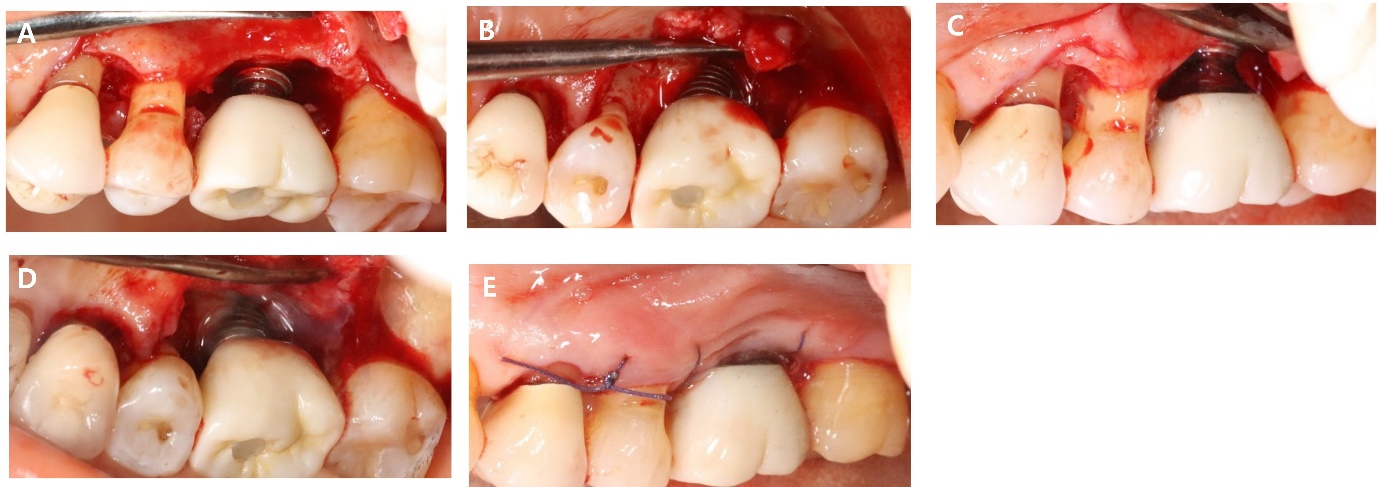
As part of non-surgical treatment, subgingival scaling, root planing, 0.12% Chlorhexidine (CHX) irrigation and topical antibiotic minocycline gel (Minocline; Dongkook pharmacy, Seoul, Korea) were applied via the gingival sulcus of the deepest pocket. Surgical procedures were performed to remove deep periodontal pockets and treat the contaminated implant surface. A sulcular incision was made starting from the mesial papilla of #24 extending to the mid-buccal area of #27. A flap was carefully reflected, and significant granulation tissue was observed around #26i, which was thoroughly debrided. Debridement included the use of titanium hand curette and scaler, mechanical CHX ball and cotton ball scrubbing, sterile saline irrigation and use of air-erythritol powder abrasive machine to decontaminate the diseased implant surfaces. There was a non-contained angular bone defect with five visible threads on the disto-palatal aspect of the implant. Enamel matrix derivatives (EMD) were applied to #24, 25, and 26i and sutured with 4-0 vicryl (Fig. 2).
The sutures were removed after one week. After eight months, bleeding on probing (BOP) was disappeared, and the probing pocket depth has been reduced in the distal area. Radiographic bone fill was observed in the distal area (Fig. 3, Table 1).
2. Case #2
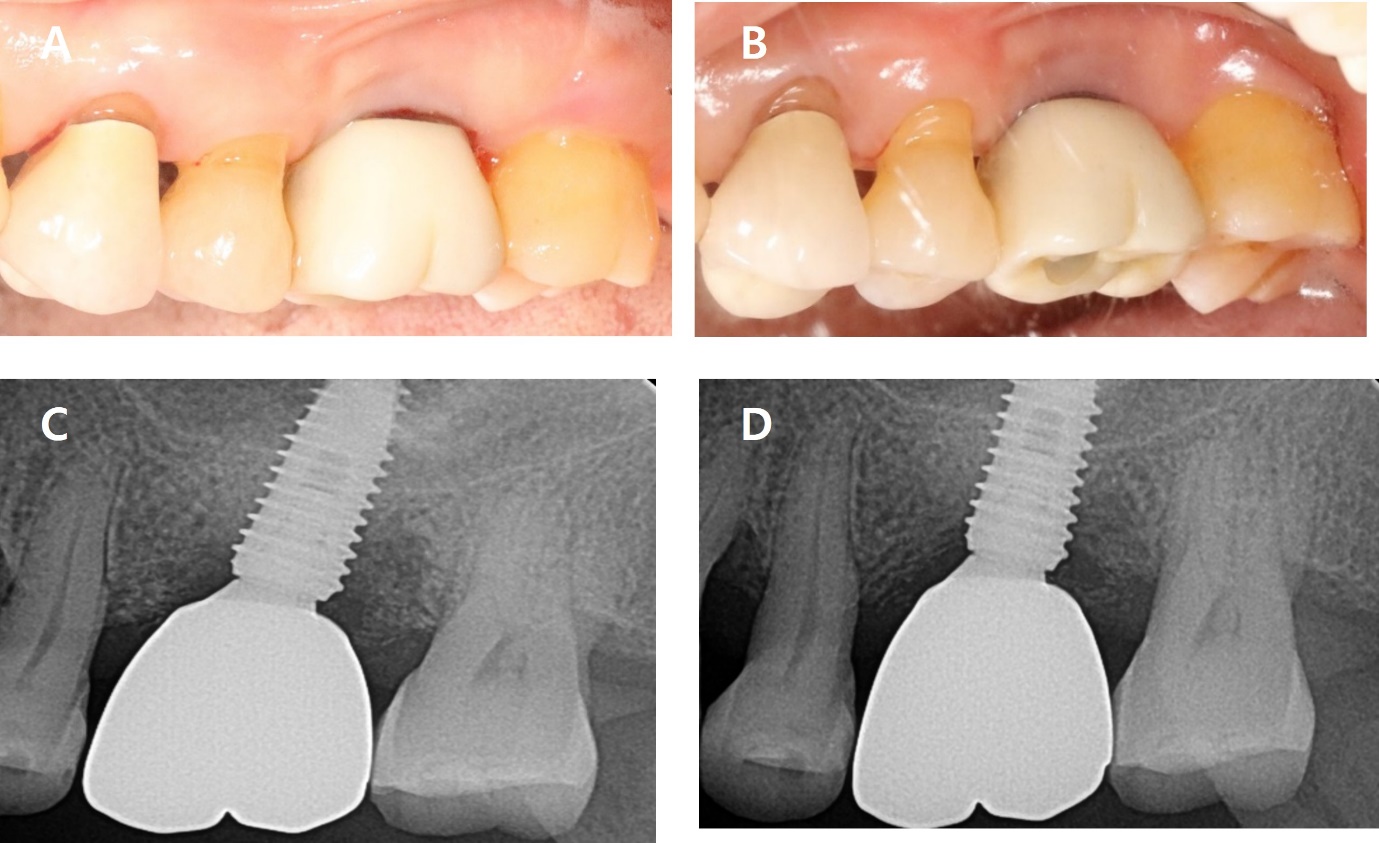
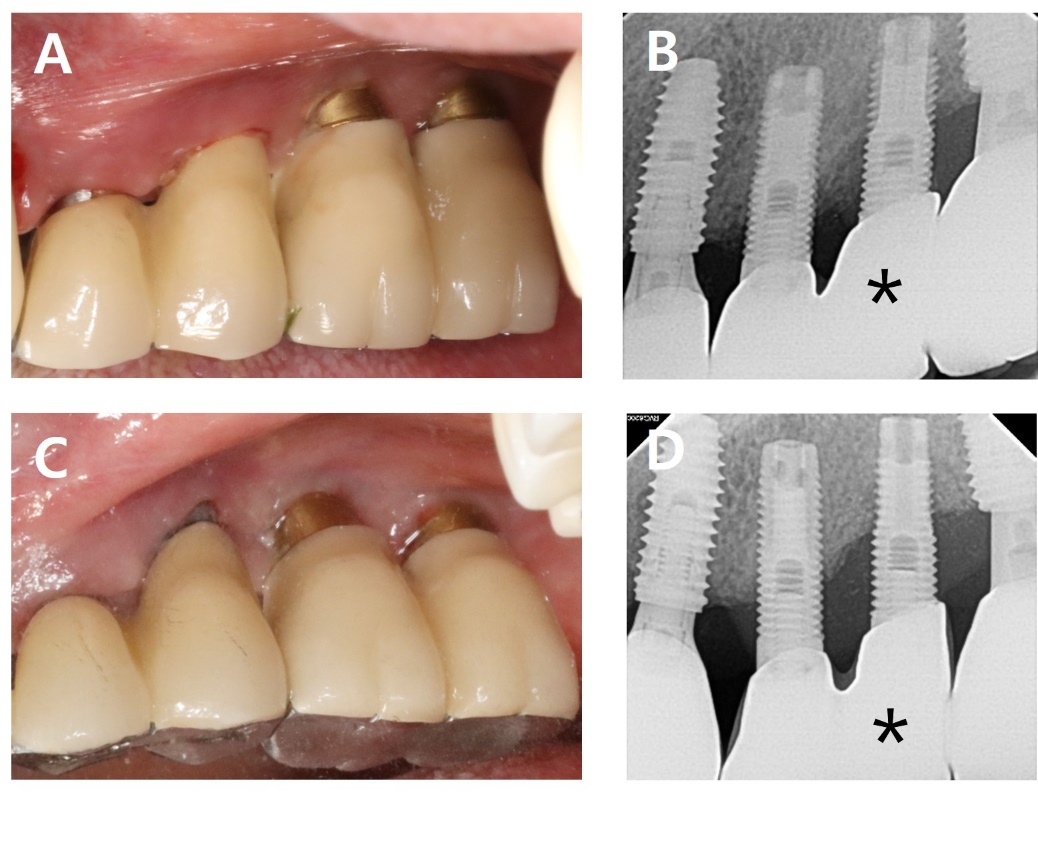
An 84-year-old man presented with bleeding at the left maxillary second premolar (#25i). Clinical examination showed an 8 mm probing depth with suppuration. The radiographic findings revealed a moderate bone defect at mesial and distal aspects of the implant, extending 5 mm from the crestal bone. A diagnosis of peri-implantitis was made for #25i (Fig. 4).
Subgingival scaling, root planing, Chlorhexidine irrigation and topical antibiotic minocycline gel (Minocline; Dongkook pharmacy) were applied via the gingival sulcus of the deepest pocket. Surgical procedures were performed to remove deep periodontal pockets and relieve recurring signs of inflammation. When the full thickness flap was reflected, and intrabony defects and residual cements were observed at implant-site. After complete removal of residual cement, thorough debridement was conducted. This include the use of titanium hand curette and scaler, mechanical CHX ball and cotton ball scrubbing, sterile saline irrigation and use of air-erythritol powder abrasive machine to decontaminate the diseased implant surfaces. A circumferential 3-wall intrabony defect with a width of 2 mm and a depth of 3 mm was observed around #25i. Xenograft (Bio-Oss; Geistlich Pharma AG, Wolhusen, Switzerland) and EMD (Emdogain; Strauman, Basel, Switzerland) were applied within the defect, followed by suturing (Fig. 5).
The sutures were removed after one week. After 10 months, BOP decreased, and pocket depth also reduced. On the radiograph, the angular bone defect disappeared and transformed into a horizontal bone shape (Fig. 6 and Table 1).
Table 1.
Comparisons of clinical indicators before and after surgery
| Case #1 | Case #2 | Case #3 | ||
| Baseline | BOP | + | + | + |
| PPD | 7 mm | 8 mm | 8 mm | |
| After surgery | BOP | - | - | - |
| PPD | 4 mm | 4 mm | 3 mm | |
| Radiographic bone gain | 1.69 | ‒1.87 | 6.42 | |
3. Case #3
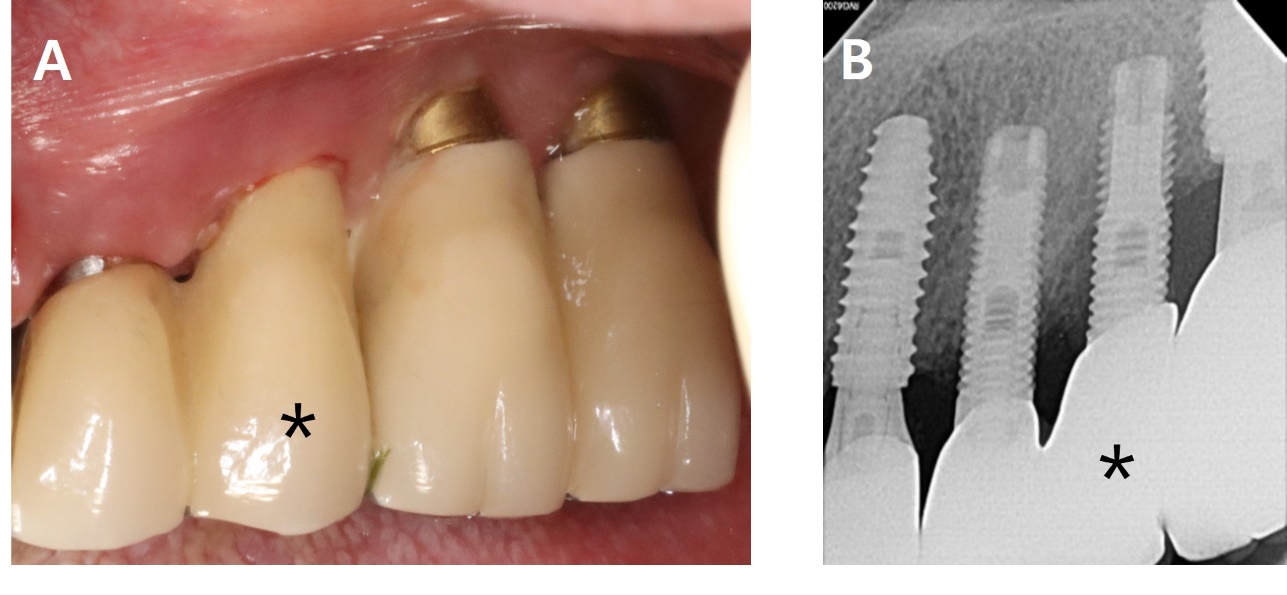
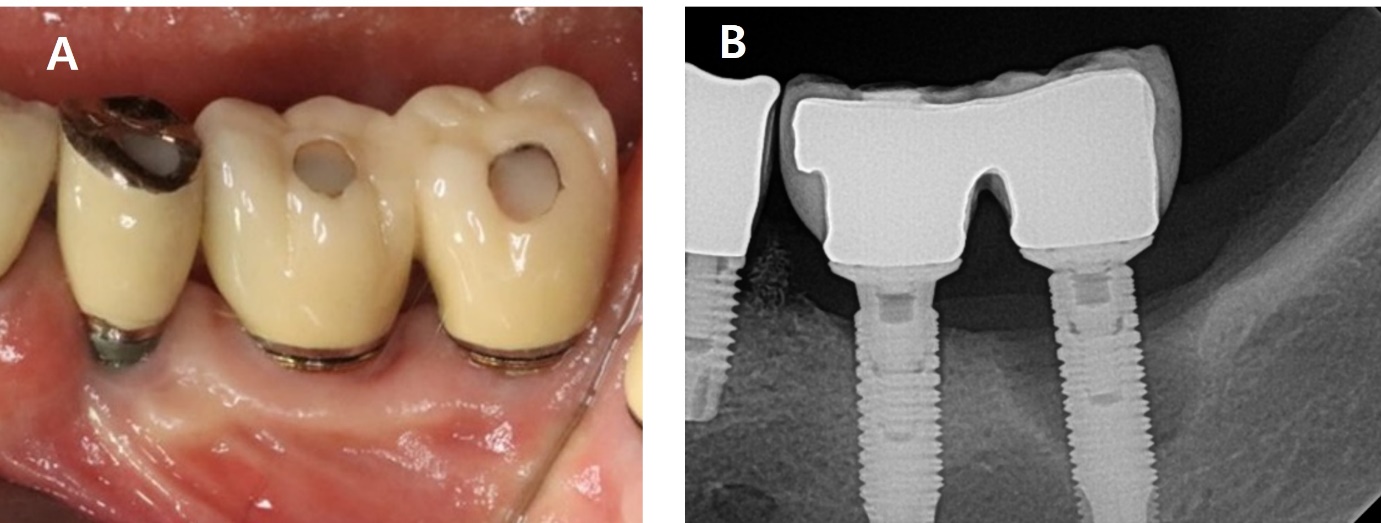
A 62-year-old woman presented with complaints of pus discharge and bleeding around the left mandibular second molar (#37i). Clinical examination showed an 8 mm probing depth with suppuration on the buccal side. The radiographic findings revealed a bone defect on the mesial, buccal and distal aspects of the implant, extending 6 mm from the crestal bone. A diagnosis of peri-implantitis was made for #37i (Fig. 7).
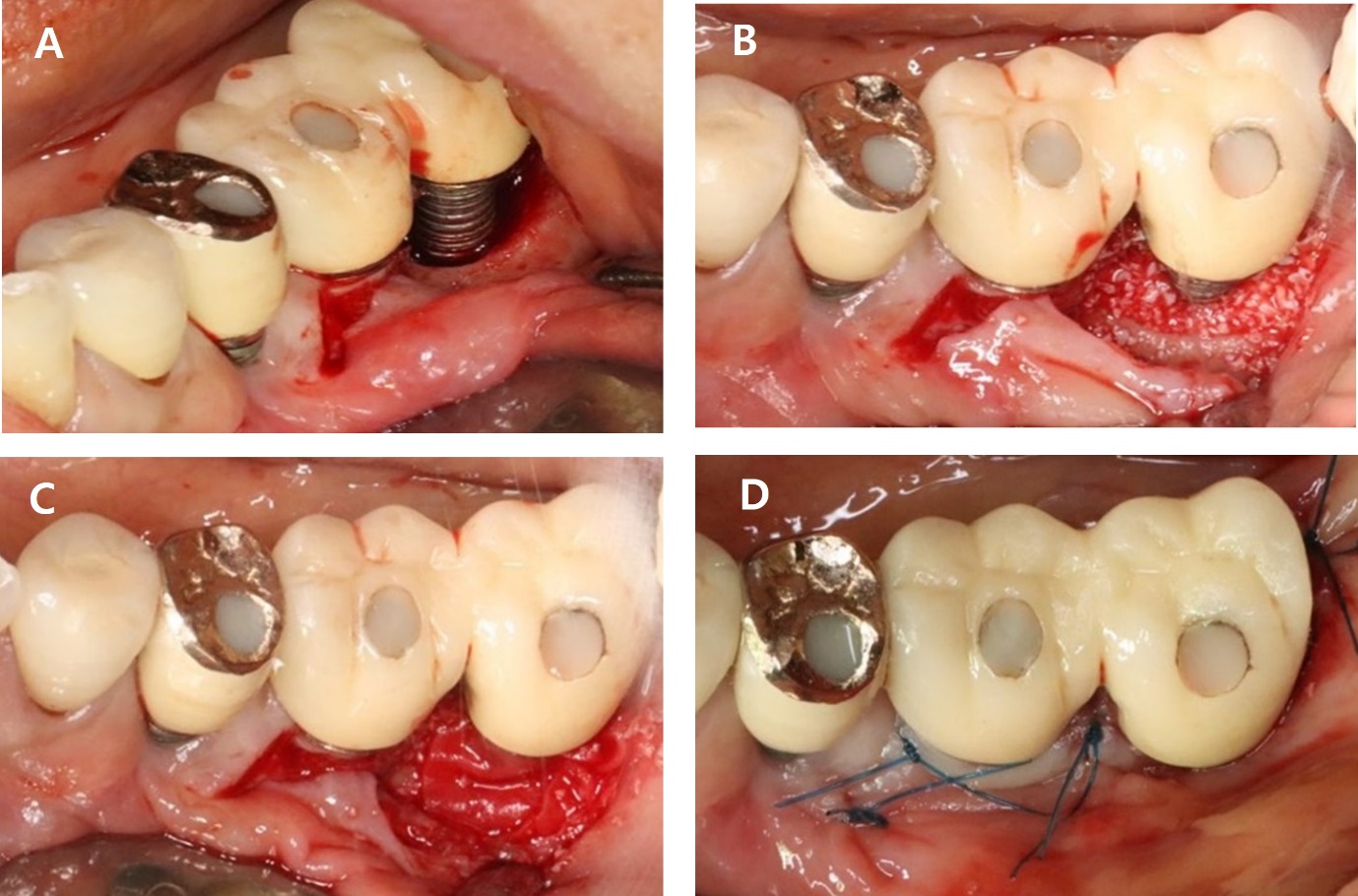
As part of non-surgical treatment, subgingival scaling, root planing, chlorhexidine irrigation and topical antibiotic minocycline gel (Minocline; Dongkook pharmacy) were applied via the gingival sulcus of the deepest pocket. Surgical procedures were performed to remove deep periodontal pockets, treat the contaminated implant surface and improve the morphology of intrabony defect. A vertical incision was made at medial aspect of #36i and sulcular incisions were made around #37i. After full-thickness flap elevation and thorough debridement, a large intrabony defect on buccal aspect of the implant was observed, measuring 3 mm in width and 6 mm in depth. The implant surface was decontaminated using titanium hand curette and scaler, mechanical CHX ball and cotton ball scrubbing, sterile saline irrigation and an air-erythritol powder abrasive machine. Within the contained intrabony defect, xenograft (Bio-Oss; Geistlich Pharma AG) and a resorbable barrier membrane (Bio-Gide; Geistlich Pharma AG) were applied, and the site was sutured (Fig. 8).
The sutures were removed after one week. After two years, BOP and probing pocket depth were reduced, Radiographic evaluation showed a substantial increase in bone fill (Fig. 9 and Table 1).
Ⅲ. Discussion
In clinical practice, peri-implantitis is diagnosed through visual examination, probing of periodontal tissues around the implant, and radiographic evaluation.7 Similar to findings in periodontal treatment outcomes,8,9 studies on peri-implantitis management report changes in clinical and radiographic parameters, with probing depth (PD) and bleeding on probing (BOP) serving as key indicators. These variables account for nearly 90% of studies on peri-implant disease management over the past 10 years.10
The importance of practical end points in active treatment has been widely studied in the field of periodontics.11 In addition to long-term outcomes, such as the absence of retreatment, tooth loss and patient-reported oral health-related quality of life, pocket closure- defined as shallow PD and absence of BOP, is strongly associated with periodontal stability.4 Similarly, for peri-implantitis management, therapeutic goals should focus on reducing PD and eliminating or minimizing BOP. The S3 level Clinical Practice Guideline by the European Federation of Periodontology recommended end points for successful surgical therapy to include shallow probing depths (≤5 mm), absence of suppuration on probing, and only minimal bleeding on probing (≤1 point).12
In clinical practice, managing peri-implantitis with non-surgical treatment is often challenging. This difficulty arises from the limited access to the implant surface, which hinders effective cleaning of the contaminated area. Therefore, surgical intervention is often required. In addition to resolving peri-implant inflammation, the goals of augmentive therapy are to: (a) regenerate the bone defect, (b) achieve reosseointegration, and (c) preserve the peri-implant soft tissue to limit regression.
Surgical augmentative therapy for peri-implantitis improved clinical and radiographic outcomes compared to baseline in three cases within a follow-up period of eight months to two years. Evaluation of the overall clinical efficacy of adjuvant augmentation therapy showed improvements in radiographic marginal bone levels, clinical attachment gains, increase in significant soft tissue recession, and reduced PD values compared to baseline. However, there are currently very few human histological case reports demonstrating re-osseointegration on previously contaminated implant surfaces following augmentation therapy.13,14This lack of evidence raises questions about whether re-osseointegration occurred in these three cases.
Various implant surface decontamination techniques have been used to achieve success in augmentation surgery for peri-implantitis. These include mechanical, chemical, laser/ozone therapy, or a combination of these. Many systematic reviews indicated that existing clinical data do not prioritize any specific implant surface decontamination protocol for augmentative approach.5 Therefore, clinicians often employ different methods to disinfect contaminated implant surfaces, as decontamination is considered critical for reconstructive treatment.
Augmentation of intrabony peri-implant defects can be performed by bone graft using bone filler particles alone (e.g., autogenous bone, xenograft, allogenic bone, and alloplastic bone substitutes) or by guided bone regeneration using additional applying of a barrier membrane, in which additional biologically active materials may be used.5 Currently, available evidence does not support the superiority of any particular material, product, or membrane in terms of long-term clinical treatment benefits of augmentative approaches. In addition to topical antibiotic application, the adjuvant uses of biologically active substances such as EMD, platelet concentrates (platelet-derived growth factor), concentrated growth factors, xenografts containing native bone morphogenetic proteins, vascular endothelial growth factor, and platelet-rich fibrin membranes have been reported to enhance reconstructive treatment outcomes for peri-implantitis.15,16,17 To achieve a successful reconstructive approach, research has mentioned the use of EMD. EMD has been successfully used for periodontal regeneration in intrabony defects. The potential beneficial effects of EMD into peri-implant defects include improving the osteoconductivity of bone grafts, providing antimicrobial properties, and promoting wound healing and tissue regeneration.18
According to the recent consensus report from the FDI World Dental Federation meeting, clinicians are advised to use submerged postoperative wound closure following augmentative therapy for peri-implantitis whenever feasible to promote physiologic healing in a biofilm-free environment.19 However, in actual clinical practice, challenges related to prosthesis removal often arise, and therefore, there is a limitation in performing augmentative therapy through a non-submerged approach in these three cases.