Ⅰ. Introduction
Ⅱ. Materials and Methods
1. Specimens Preparations
2. Surface Morphology Analysis
3. Specimen Preparation and Shear Bond Strength Test
4. Statistical Analysis
Ⅲ. Results
Ⅳ. Discussion
Ⅴ. Conclusion
Ⅰ. Introduction
As social conditions and welfare improve around the world, human life expectancy is increasing.1 In order to have a healthy old age, having healthy oral health is a prerequisite.2 However, data from the National Institute of Dental and Craniofacial Research (NIDCR, National Institutes of Health, United States) have reported that about 90% of over 70 years old people have a periodontal-related diseases.2, 3, 4 Periodontal disease causes clinically diverse symptoms like a gingival hemorrhage, swelling, and eventually tooth loss.2, 5 To replace the missing teeth, implant surgery is being increasingly performed and popularized in various situations.6
Among the method of the implant prosthetic treatments, a method of using titanium abutment and using a metal core for the upper prosthesis had been treated a lot, but as aesthetic demands increase, the use of the zirconia, which is capable of reproducing the tooth color, as the upper prosthesis is increasing.7 Various methods to increase the bonding strength between zirconia and titanium have been reported.8, 9, 10 However, all of the previous studies were methods of increasing bonding strength via treating airborne- particle abrasion to zirconia.8, 9, 10
Treating airborne-particle abrasion with Al2O3 on zirconia surface is the most widely used method to increase micromechanical retention of zirconia surface.11 However, the mechanical effect of particle abrasion can accelerate the failure by inducing micro-cracks on the zirconia surface; furthermore, it can reduce the zirconia flexural strength by up to 30%.12, 13
To overcome the limitation of previous introduced methods, a novel zirconia surface treatment method, sintering after applying ZrO2 slurry with carbon nanoparticle to pre-sintered zirconia surface, has been introduced to improve the bond strength by increasing zirconia surface porosity and roughness. Nano-size ZrO2 particles can be connected to zirconia surface through hydrogen bonding under basic conditions, and carbon particle can produce reticulated porous zirconia after sintering according to previous studies.14, 15
This study was conducted to evaluate roughness of zirconia surface after applying ZrO2 slurry to the pre-sintered zirconia surface before sintering process, and to test the shear bond strength between zirconia and self-adhesive resin cement. The concentration of ZrO2 slurry may be an important factor to change the micro-structure of zirconia surface, and the changed microstructure can affect the bond strength between zirconia and self-adhesive resin cement.
Ⅱ. Materials and Methods
1. Specimens Preparations
Eighty cylindrical pre-sintered zirconia discs of 20 mm in diameter and 5 mm in thickness (Zpex Smile, Tosoh, Tokyo, Japan) and ZrO2 slurry (ZirADD, PNUADD, Busan, Korea) were prepared. All specimens were divided into four groups, including control (no treatment) and three groups, according to ZrO2 slurry concentration: a slurry containing 1.5 g nano-sized ZrO2 powder compared to 100 g of distilled water as a solvent (ZA15), a slurry containing 3.0 g ZrO2 powder compared to 100 g of distilled water as a solvent (ZA30), and a slurry containing 5.0 g ZrO2 powder compared to 100 g of distilled water as a solvent (ZA50) were prepared for test groups. After all test groups were treated with different concentration of ZrO2 slurry, they were sintered at 1,530℃ for 12 hours (n = 20 in each group). Half of specimen were used for surface roughness test, and the other half were tested for shear bond strength.
2. Surface Morphology Analysis
After surface treatment, zirconia surface was inspected at 5,000X magnification using scanning electron microscopy (SEM) (Gemini SEM300, Carl Zeiss, Oberkochen, Germany). To measure the surface roughness (Ra in µm), profilometer (Mitutoyo Surftest SV 2000, Mitutoyo, Kanagawa, Japan) was used. The surface roughness of the specimen was measured for a distance of 4.8 mm.
3. Specimen Preparation and Shear Bond Strength Test
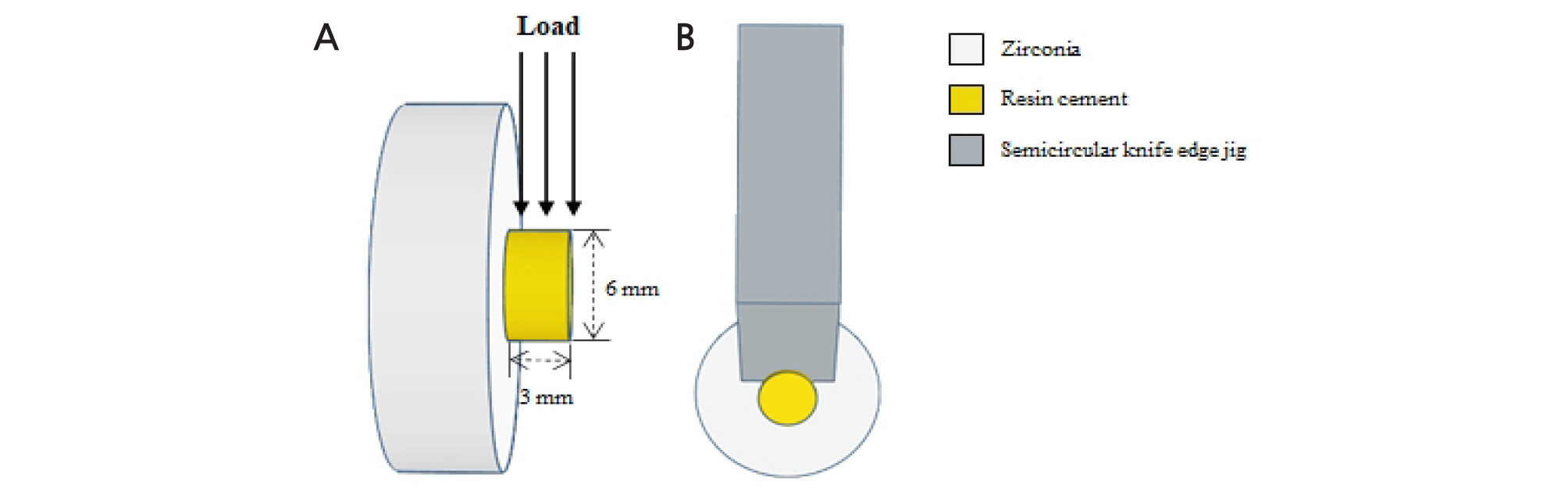
A cylindrical metal mold having an inner diameter of 6 mm and a thickness of 3 mm was placed on the surface-treated zirconia disc. Self-adhesive resin cement (MAZIC CEM, Vericom, Anyang, Korea) with 10- methacryloyloxydecyl dihydrogen phosphate (MDP) was filled in the metal mold (8 mm outer diameter, 6 mm inner diameter, and 3 mm height) and light cured for 20 seconds on the side and 20 seconds from the top. After light curing, all specimens were stored in distilled water at room temperature for 24 hours before shear bond strength test. Shear bond strength test was performed using a testing machine (OUT-05, Oriental TM Corp., Gyenonggi-do, Korea) at a constant crosshead speed 1.0 mm/ min until failure (Fig. 1).
4. Statistical Analysis
SPSS software ver. 25.0 (SPSS Inc., Chicago, USA) was used for the statistical analysis and all measured values were evaluated with the 5% of the level of significance. Surface roughness values were analyzed by the Kruskal-Wallis tests with post hoc pairwise Mann-Whitney analyses. And the results of the shear bond strength were analyzed using one-way analysis of variance (ANOVA) and Tukey’s post-hoc test.
Ⅲ. Results
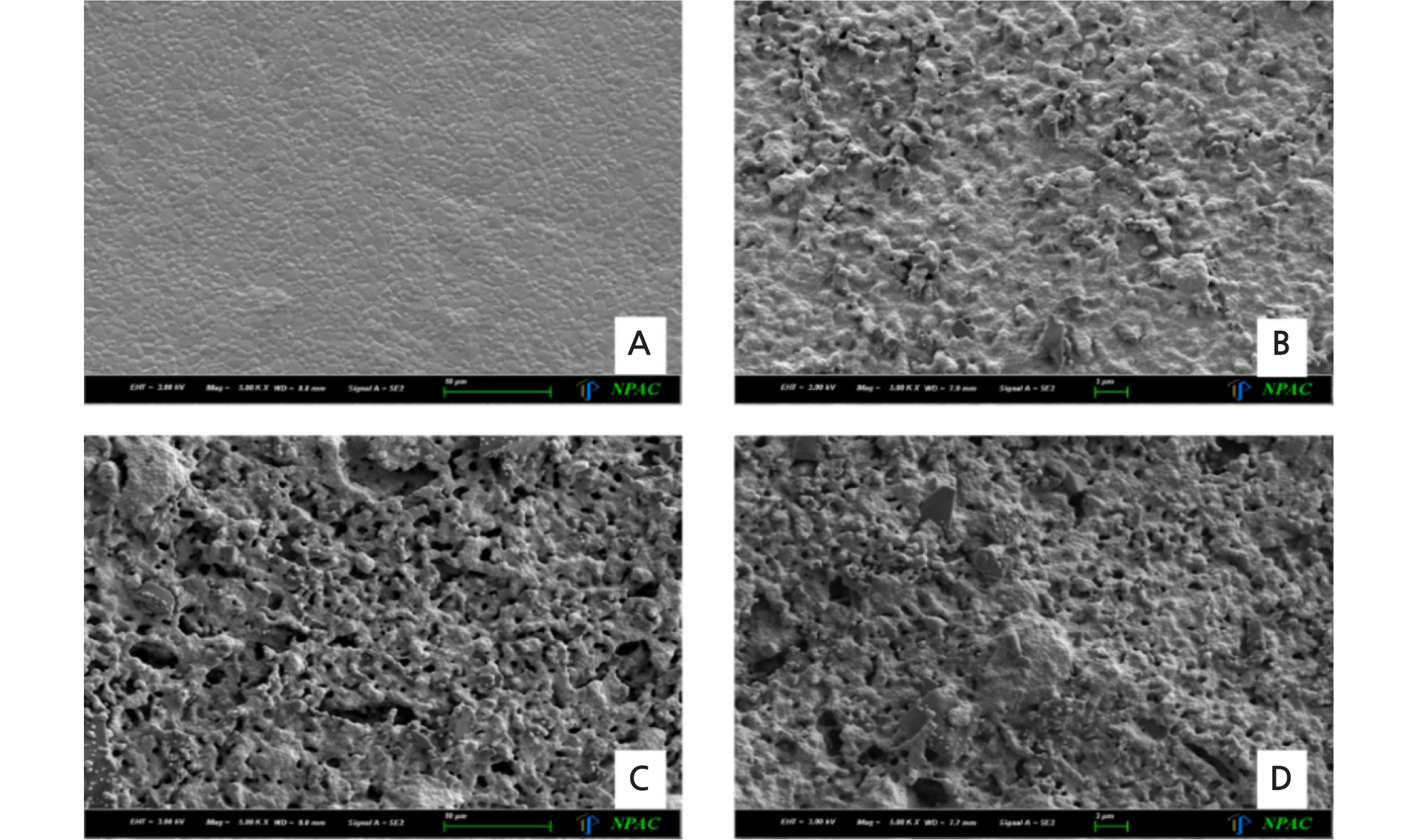
Surface roughness images of each experimental group captured by SEM were shown in Fig. 2. The control group showed a typical untreated surface and the test groups showed increasing surface roughness with irregularity.
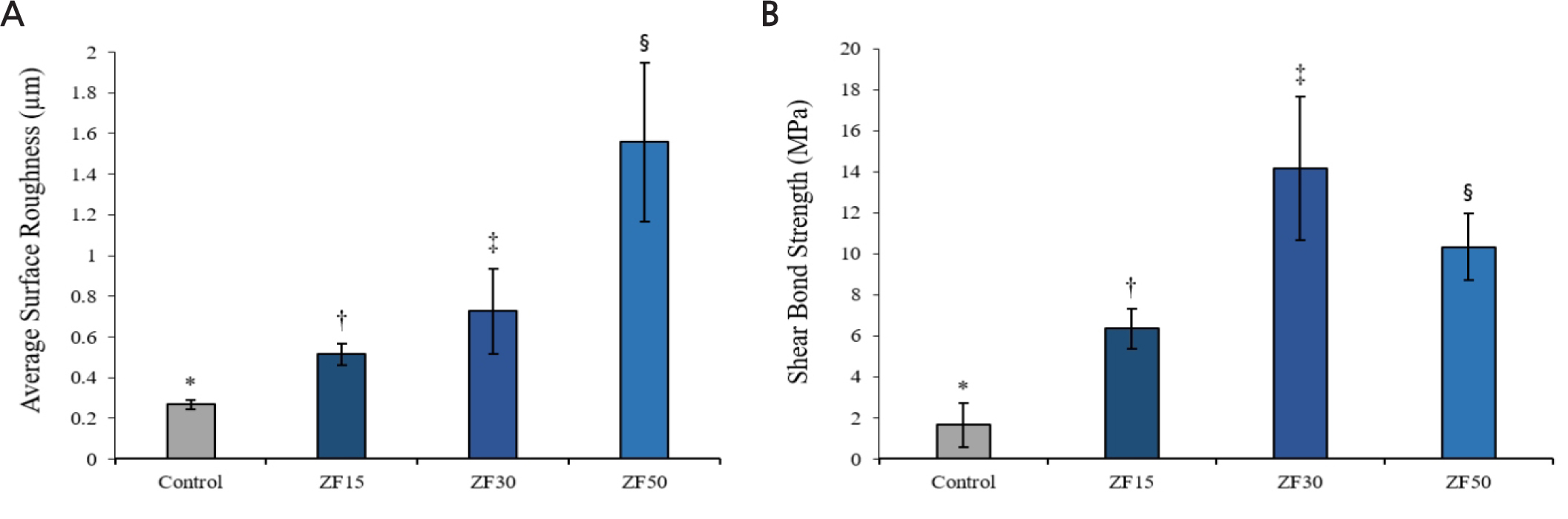
The mean surface roughness value (Fig. 3A) and shear bond strength values (Fig. 3B) of each group are shown. According to the test, the means of surface roughness values (Ra) of all groups were as following: The values of control, ZA15, ZA30 and ZA50 groups were 0.27 ± 0.02, 0.51 ± 0.05, 0.73 ± 0.21, 1.56 ± 0.39, respectively (Table 1). ZA50 group showed the highest surface roughness value compared to others (p < .05). And the mean shear bond strength values (MPa) were significantly different between all tested groups (p < .05): The values of control, ZA15, ZA30 and ZA50 groups were 1.66 ± 1.07, 6.35 ± 0.98, 14.15 ± 3.49 and 10.32 ± 1.63, respectively (Table 2).
Ⅳ. Discussion
The bond strength between zirconia and resin cement can be affected by pre-treatment on zirconia surface for mechanical interlocking.16 In this study, porosity and micromechanical irregularities were formed on zirconia surface by applying ZrO2 slurry with carbon nanoparticle on pre-sintered zirconia surface. Through the SEM images and surface roughness results, average surface roughness has increased considerably after applying high concentration of ZrO2 slurry on pre-sintered zirconia surface. In a previous study, zirconia particle can have negative charge through absorbing OH - ions under basic condition, and negative charged zirconia particle bonds with water molecules as hydrogen boding, and zirconia particles can interconnect and agglomeration with each other.15
When ZrO2 slurry was applied to pre-sintered zirconia surface, shear bond strength with self-adhesive resin cement were measured higher than control group. A previous study reported that self-adhesive resin cement penetrates and interlocks with the network of nano-porosities of zirconia surface.17 The method using ZrO2 slurry with carbon nanoparticle was expected be helpful to increase bonding strength of resin cement by penetration and interlocking of resin cement. Furthermore, the surface roughness value of ZA50 was highest among experimental groups, whereas highest shear bond strength value was shown in ZA30. It seems that the too many zirconia particles in ZA50 group could not make perfect unification on the surface for increasing the roughness. These results are similar with other previous study that was reported the surface irregularities without undercut can decrease the bond strength after prolonged period.18 However, additional clinical studies are needed to certify interlocking between ZrO2 slurry applied coating layer and self-adhesive resin cement.
As this study was planned as a pilot study to evaluate surface roughness and shear bond strength, aging process like thermocycling was not included. Therefore, aging/stressing studies in vitro and long term in vivo studies are further required.