Ⅰ. Introduction
Ⅱ. Case Report
1. First surgery: extraction of all remaining maxillary dentition and extruded mandibular teeth
2. Second surgery: bilateral maxillary sinus bone graft and implant placement on the mandible after extraction of the remaining teeth
3. Third surgery: implant placement on the maxilla
4. Delivery of implant-supported fixed prosthesis
Ⅲ. Discussion
Ⅳ. Conclusion
Ⅰ. Introduction
Full mouth rehabilitation for edentulous patients requires attention from the initial stage of diagnosis and treatment planning to the final stage of restoration and postoperative management. Multiple factors should be comprehensively considered before implant surgery, including the location of placement, necessity for bone graft, time of placement and loading, and type of restoration.
Patients with chronic systemic diseases may have difficulties in the rehabilitation of multiple missing teeth with implants due to the risk of complications, including bleeding tendency and abnormal bone healing after dental surgery.
Recently, a surgical guide using cone-beam computed tomography (CBCT), a dental cast model, and oral scan data has improved the accessibility of multiple and full-mouth implant treatment because it can shorten the operating time and simplify the subsequent restoration process. However, the implant surgical guide cannot be designed properly in some patients with difficulty in undergoing CBCT due to systemic disease.
We report the case of a patient with end-stage renal disease (ESRD) and diabetes mellitus (DM) with complicated polyneuropathy who was successfully treated with a protocol of full mouth rehabilitation including bilateral maxillary sinus bone graft and immediate mandibular implantation.
Ⅱ. Case Report
A 50-year-old man presented to our hospital with an overall recovery of masticatory function. The patient had been diagnosed with DM 10 years prior, underwent insulin treatment, and received hemodialysis treatment three times weekly. At his presentation to our hospital, hematology evaluations revealed a hemoglobin concentration of 10.4 (normal 13–17) g/dL and a hematocrit level of 31.9 (normal 36 to 48)%. The blood chemistry values were as follows: blood urea nitrogen (BUN), 57.7 (normal 7–25) mg/dL and creatinine, 5.03 (0.5–1.2) mg/dL. His glomerular filtration rate (GFR) was 12.04 mL/ min per 1.732 and his hemoglobin A1c (HbA1c) level was 7.5 (normal 4.3–6.3)%.
The patient was unable to walk without assistance due to severe foot drop or inability to raise the front part of his foot owing to diabetic polyneuropathy. In addition, the patient was taking medications for diabetic retinopathy and high blood pressure.
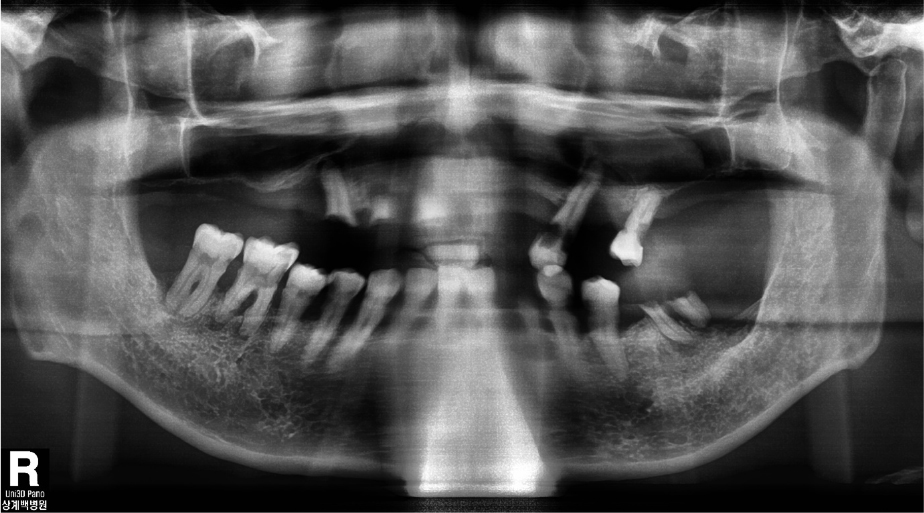
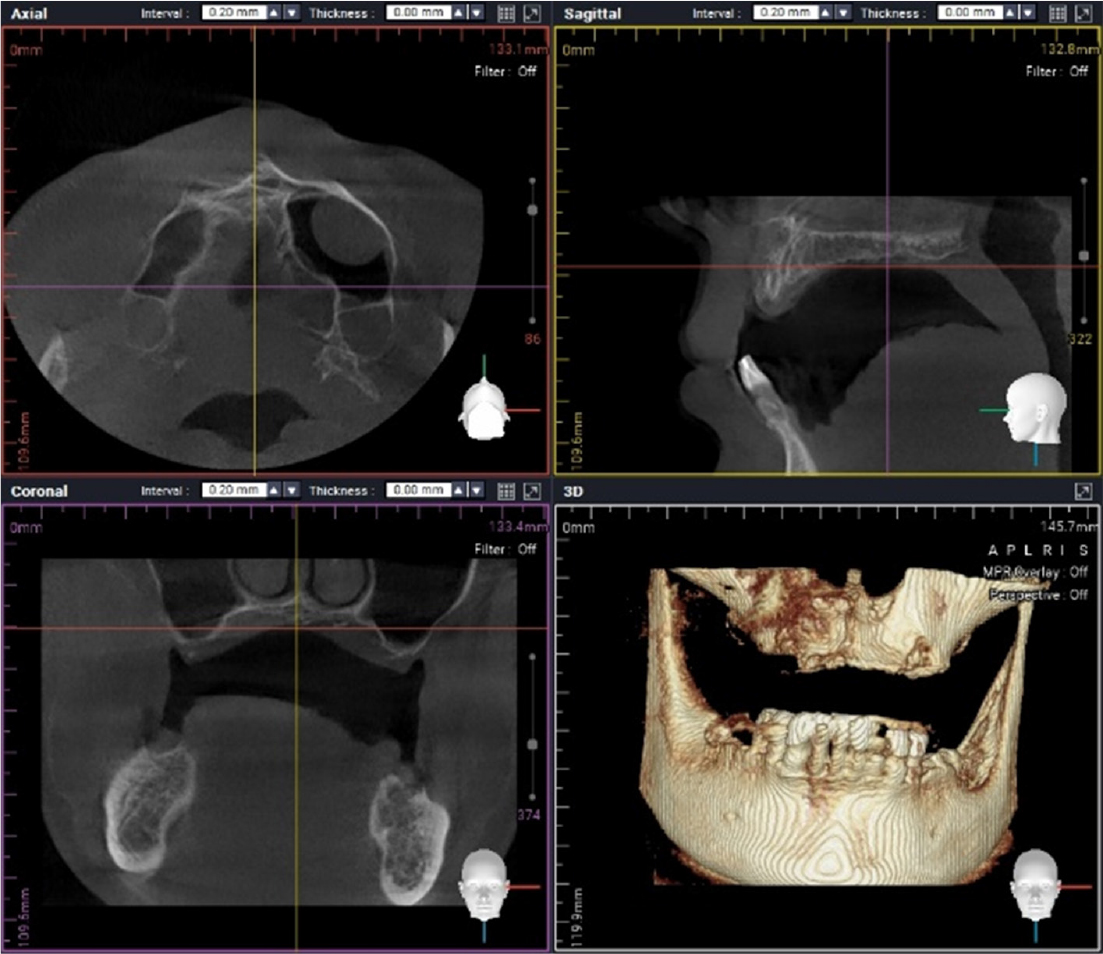
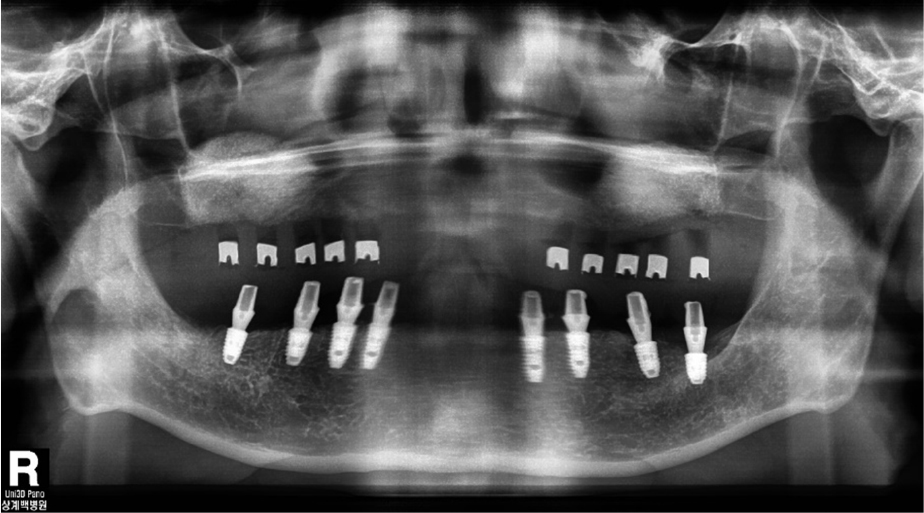
Oral examination of the patient revealed several dental caries and residual roots. The maxillary and bilateral posterior alveolar bones had undergone severe vertical resorption, requiring restoration with extraction of all dentition (Fig. 1). The patient desired implant treatment. After consulting with his nephrologist, a total of three surgeries were planned according to the patient’s general systemic condition. Full rehabilitation with an implant-supported fixed restoration was also planned. Guided surgery was attempted using a minimally invasive surgical method; however, due to diabetic neuropathy, it was difficult to obtain an accurate CBCT image (Fig. 2).
A series of three surgeries was performed under local anesthesia after the prescription of prophylactic antibiotic medication (ceftriaxone, 1 g administered intravenously). After each surgery, oral antibiotics (cefaclor 250 mg/bid), analgesics (acetaminophen 500 mg/bid) were prescribed for 5 days, and nasal decongestants (Pseudoephedrine 30 mg/tid) were prescribed for 5 days after second surgery, while chlorhexidine mouth rinse was recommended for 7 days.
The surgical procedures performed at each stage were as follows:
1. First surgery: extraction of all remaining maxillary dentition and extruded mandibular teeth
The remaining maxillary and extruded (#46 and #47) teeth were extracted under local anesthesia. A tissue conditioner was then applied to a temporary denture fabricated in advance for hemostasis and gingival recovery.
2. Second surgery: bilateral maxillary sinus bone graft and implant placement on the mandible after extraction of the remaining teeth
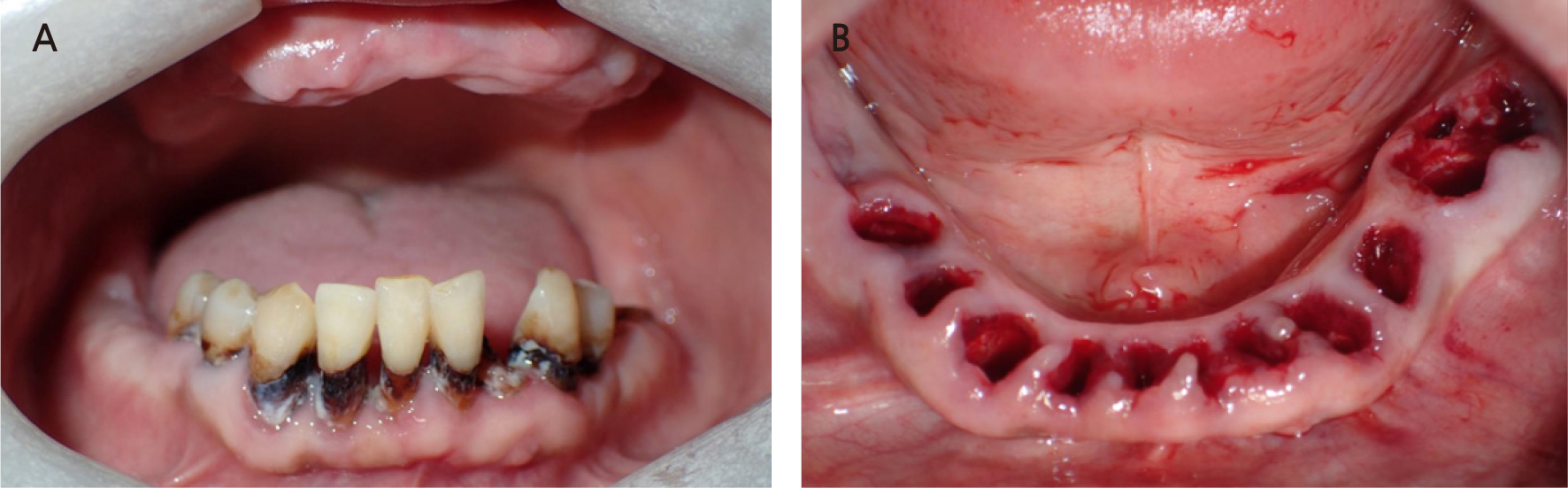
The second surgery was performed 2 months after the first surgery (Fig. 3A). The remaining teeth (#36, #34, #33, #32, #31, #41, #42, #42, #44, and #45) were extracted (Fig. 3B), and six implants (Toplan, Seoul, Korea) were placed immediately at #33, #34, #36, #43, #44, and #45. Two implants (Toplan) were placed in edentulous areas of #37 and #46. After implant placement, a 1:1 mixture of allogenic bone (DO BONE™, CGBIO, Seoul, Korea) and xenogeneic bone (Bio-Oss®, Geistlich, Wolhusen, Switzerland) was grafted onto the gap between the implant and labial bone in areas #33, #34, #43, and #44. The primary stability was achieved properly. The initial torque and resonance frequency analysis (RFA) values measured at the end of the surgery are shown in Table 1.
Table 1.
RFA values (ISQ) at the buccal (labial), lingual, mesial, and distal sides of the implants by Osstell®ISQ at 3 months
| Position |
Implant size (mm) |
Insertion torque (Ncm) | Initial ISQ value | ISQ value at POD 3 months |
| #33i | 4.0 × 10.0 | 15 | 73/73/75/75 | 80/80/80/80 |
| #34i | 4.5 × 10.0 | 25 | 80/84/84/84 | 85/85/75/75 |
| #36i | 5.0 × 8.0 | 25 | 81/80/79/79 | 84/85/85/85 |
| #37i | 5.0 × 7.0 | 55 | 83/81/81/80 | 87/85/86/86 |
| #43i | 4.0 × 10.0 | 15 | 72/72/77/77 | 75/75/82/81 |
| #44i | 4.5 × 10.0 | 20 | 74/74/74/79 | 86/86/86/86 |
| #45i | 4.5 × 8.0 | 15 | 68/68/65/65 | 84/79/79/79 |
| #46i | 5.0 × 8.0 | 55 | 80/80/71/71 | 77/77/86/86 |
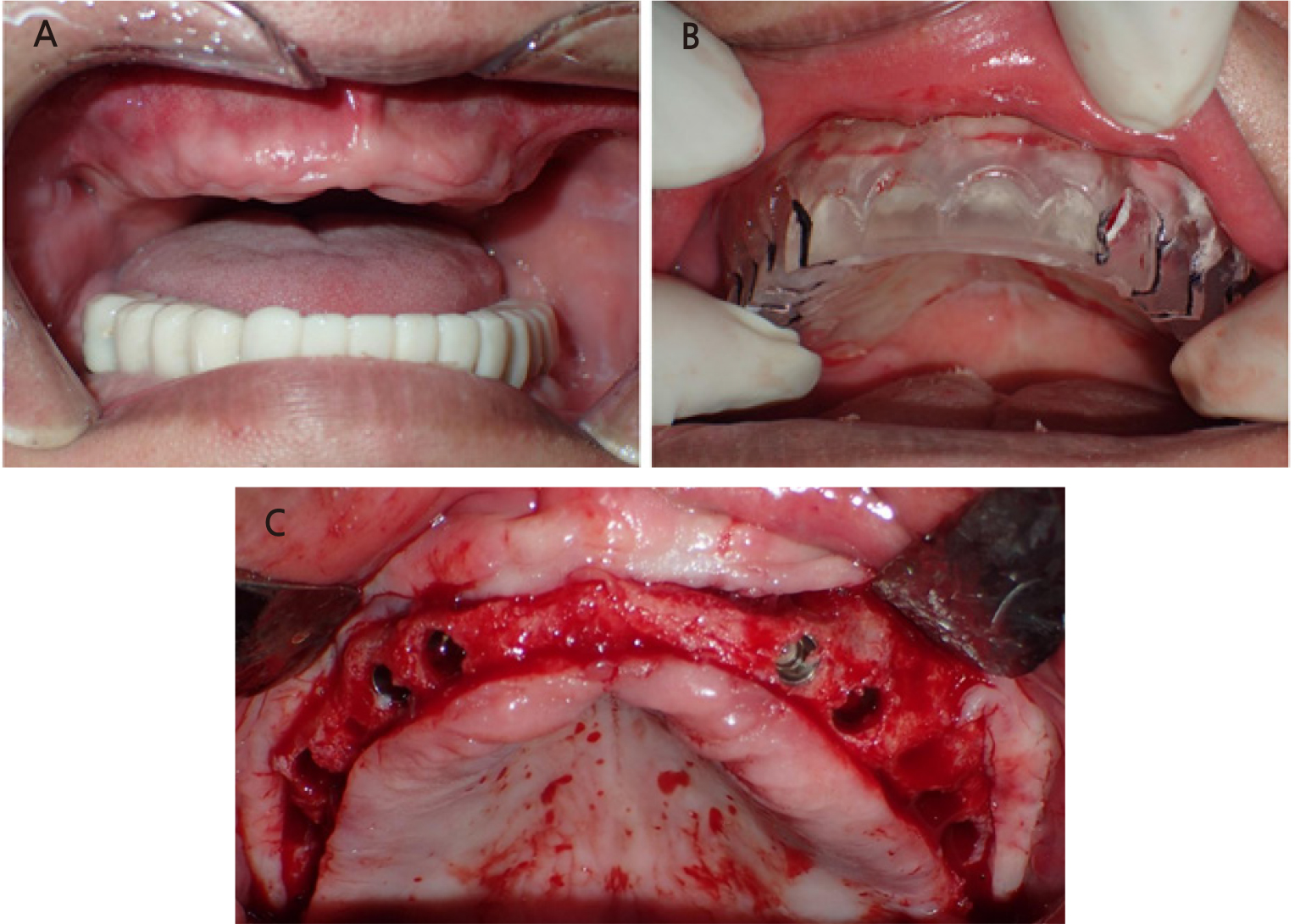
Significant alveolar bone loss was observed around the maxillary posterior area, which had a residual alveolar bone height of approximately 2 mm. Sinus bone grafting was performed using the lateral approach (Figs. 4 and 5). A 1:1 mixture of xenograft (Bio-Oss®, Geistlich) and allograft (DO BONE™, CGBIO) was used as the bone graft material. No perforation was observed during the maxillary sinus mucosal elevation.
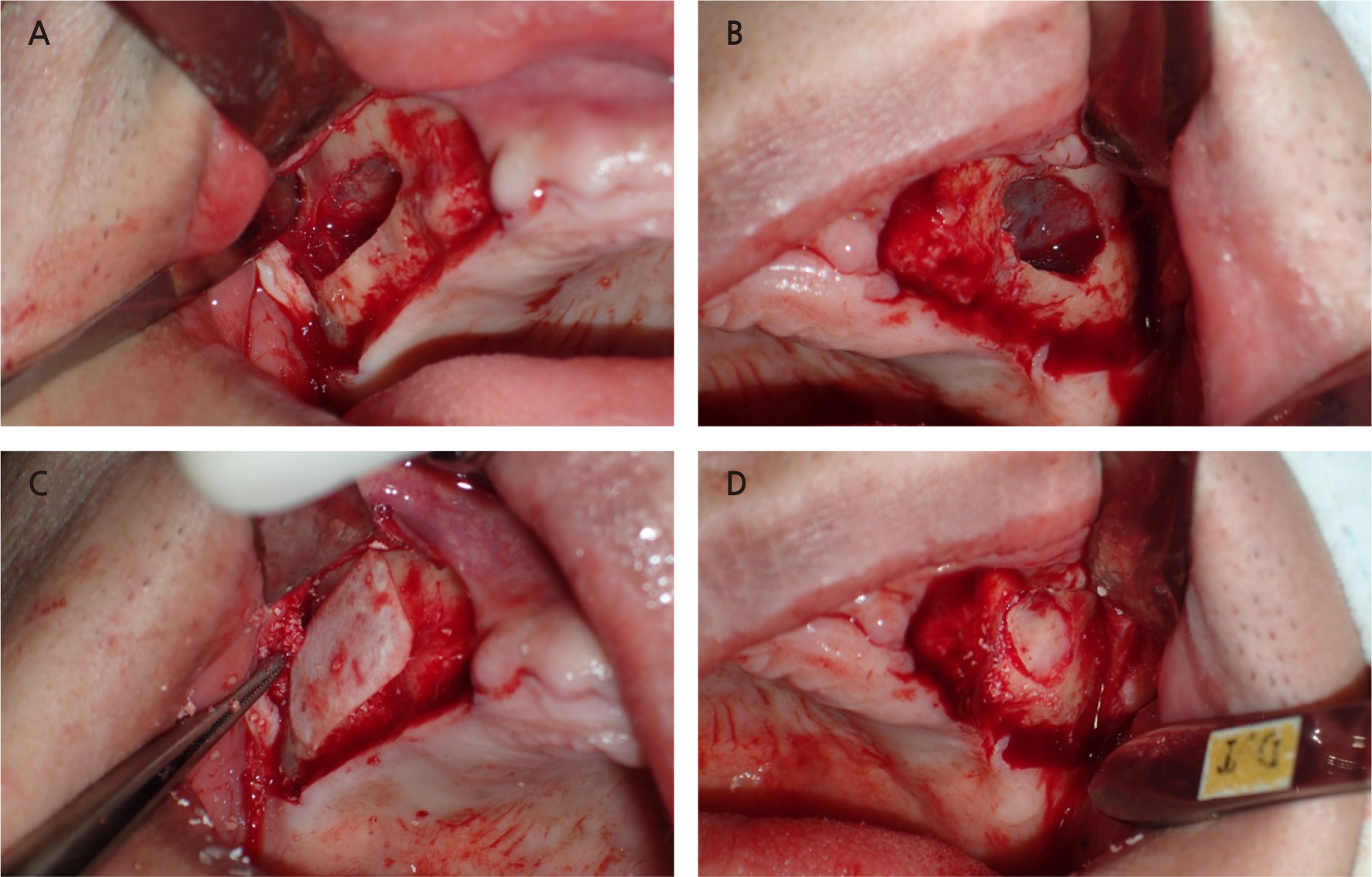
Fig. 5.
Clinical photo showing the lateral approach for sinus floor elevation. (A) A window in the lateral sinus wall on the right maxilla, (B) A window in the lateral sinus wall on the left maxilla, (C) An absorbable collagen membrane over the grafting material, (D) Bony plate repositioned over the grafting material.
Immediate mandibular dentures were delivered postoperatively. The inner surfaces of the maxillary and mandibular temporary dentures were periodically replaced after application of the tissue conditioner. Three months after the healing period, RFA values were recorded (Table 1), and the implant showed stable fixation. The abutment screws were torqued into place and a temporary bridge was delivered for 4 months of conventional loading after the second surgery.
3. Third surgery: implant placement on the maxilla
Placement of eight implants (Toplan) was performed 6 months after maxillary sinus bone grafting. As mentioned earlier, owing to the difficulty of CBCT, a panoramic image was taken with a conventional radiological guide before the third surgery (Fig. 6). The conventional surgical guide was placed stably in the maxilla and the surgery was completed in a non-submerged implantation (Fig. 7). The initial stability was appropriate during implantation; the implantation torque and RFA values are listed in Table 2. RFA values obtained after 2 months of healing suggested that osseointegration was well achieved. The abutment screws were torqued into place, and a temporary bridge was delivered for conventional loading, 3 months after the third surgery.
Table 2.
RFA values (ISQ) at the buccal (labial), lingual, mesial, and distal sides of the implants by Osstell®ISQ at 2 months
| Position |
Implant size (mm) |
Insertion torque (Ncm) | Initial ISQ value | ISQ value at POD 2 months |
| #13i | 3.6 × 10.0 | 30 | 79/69/80/80 | 71/58/71/71 |
| #14i | 4.0 × 10.0 | 25 | 72/72/79/79 | 70/79/79/79 |
| #16i | 5.0 × 10.0 | 25 | 74/80/77/79 | 75/73/72/71 |
| #17i | 5.0 × 10.0 | 37 | 79/79/71/80 | 80/71/75/78 |
| #23i | 3.6 × 8.0 | 30 | 65/56/65/65 | 66/77/77/77 |
| #24i | 4.0 × 10.0 | 25 | 68/76/76/76 | 80/80/80/80 |
| #26i | 5.0 × 10.0 | 35 | 70/72/72/72 | 75/75/75/75 |
| #27i | 5.0 × 10.0 | 37 | 66/67/67/66 | 76/71/67/78 |
4. Delivery of implant-supported fixed prosthesis
The insertion torques of six out of eight implants in the mandible were <30 Ncm, while those of the other two implants (#36 and #37) were >30 Ncm. To ensure implant stability, the RFA values were measured 3 months after the final surgery (Table 1). Conventional loading was performed after 4 months with a fixed provisional prosthesis.
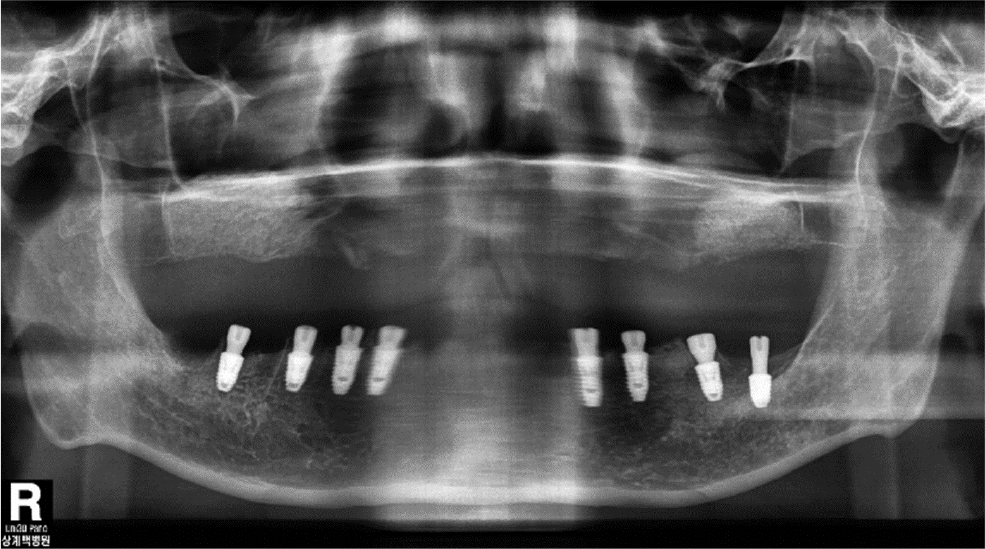
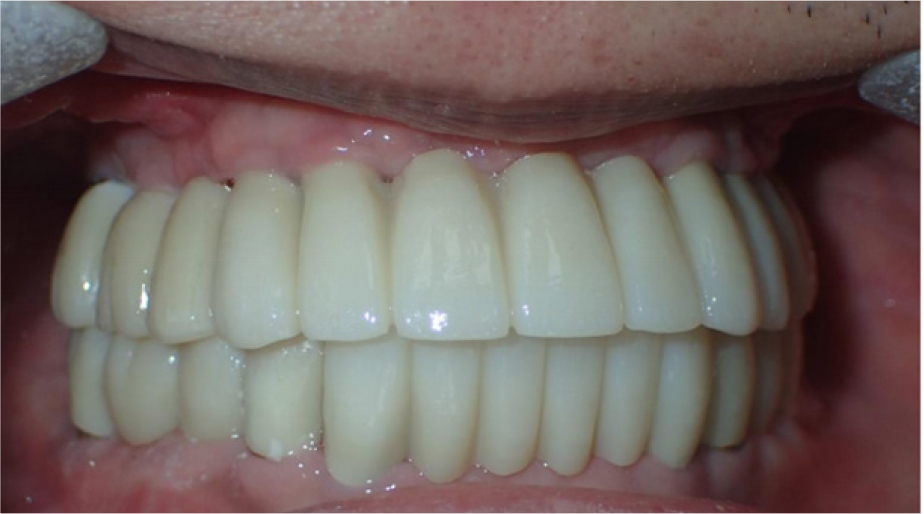
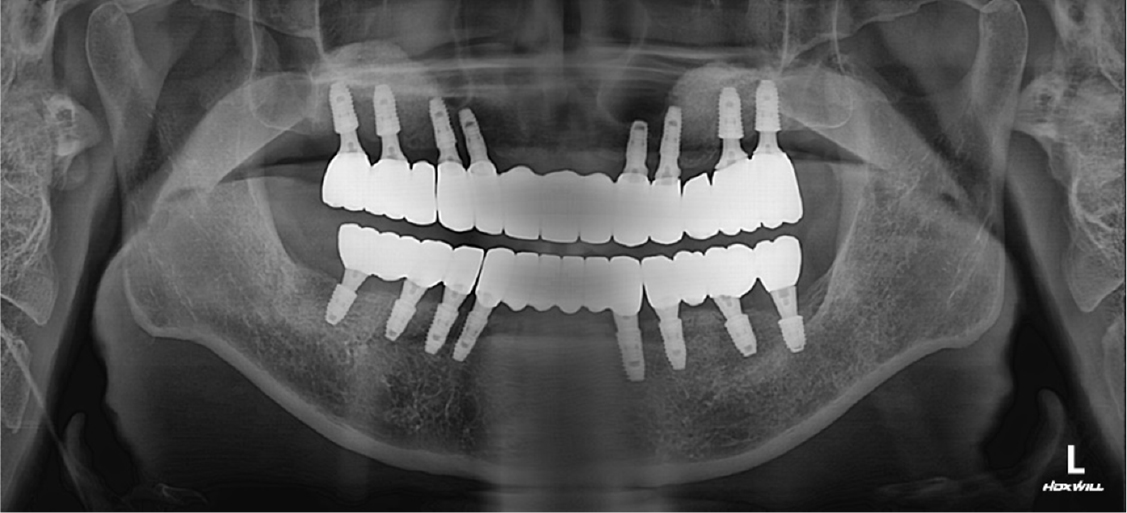
After obtaining an implant-level impression of the maxilla, a custom abutment was mounted using computer-aided design/manufacturing (CAD/CAM). The final restoration was completed with segmented zirconia bridges. For the mandibular prostheses, an abutment-level impression was made on the ready-made abutment used for the provisional bridge, and the final restoration was installed with segmented zirconia bridges (Fig. 8). Follow-up clinical and radiological examinations performed at 1 year showed no abnormal findings (Fig. 9). Finally, the patient was satisfied with the improved masticatory function and esthetics.
Ⅲ. Discussion
Renal replacement therapy is indicated for patients with symptoms of uremia, severe hyperkalemia, metabolic acidosis, bleeding tendency, or GFR <10 mL/min per 1.732. Patients receiving renal replacement therapy, including dialysis, are particularly vulnerable to infection, 1 and those undergoing hemodialysis during renal replacement therapy may have platelet dysfunction due to dialysis. Moreover, the use of anticoagulants such as heparin for hemodialysis introduces a risk of delayed bleeding during invasive dental treatment.1 Therefore, invasive surgery for chronic kidney disease (CKD) requires the prevention or treatment of continued bleeding; moreover, thorough preparation is essential to prevent infection during dental surgery.
In the present case, preoperative prophylactic antibiotics were used to control the infection, with dose adjustments according to the GFR level. Patients undergoing dialysis do not require additional antibiotics after dialysis. Since these patients may have reduced excretion rates, the blood concentrations of antibiotics should be measured to determine the need for dose adjustment.
Nonsteroidal anti-inflammatory drugs (NSAIDs) are commonly used when analgesics are required after surgery. However, NSAIDs should be avoided in patients undergoing dialysis, as they are at a higher risk for gastrointestinal bleeding. In particular, NSAIDs cause vasoconstriction and reversible kidney impairment in patients with decreased renal function; therefore, alternative analgesics should be considered.
In addition to the characteristics of delayed hemostasis, patients with CKD may have oral manifestations such as dry mouth, increased calculus and plaque deposition, decreased cancellous bone trabeculae, and bad breath due to endocrinological imbalance, uremic metabolic disorders, and immunological changes.2
The blood chemistry value commonly used to evaluate renal function is the measurement of serum creatinine level. Normally, no critical changes occur in creatinine levels (normal 0.5–1.2 mg/dL) because they depend on the stability of skeletal muscle mass. Increased serum creatinine above the normal range indicates decreased renal function. The patient in this case showed a serum creatinine level of 5.03 mg/ dl and GFR of 12.04 mL/min per 1.732 at his first visit. Because the patient was on dialysis, the laboratory results were variable, and the serum creatinine level tended to decrease from about 7.5 to 5.0 mg/dL. Patients undergoing hemodialysis are recommended to undergo dental surgery on the day after dialysis. During hemodialysis, unreleased toxins are removed, the intravascular volume is increased, and heparin metabolism is in an ideal state.3
When multiple implant surgeries are required for patients with multiple tooth loss, flapless surgery could be helpful due to the reduced the surgery invasiveness, risk of bleeding, and operating time.4 Performing flapless surgery requires an understanding of the condition of the alveolar bone below the gingiva to select the appropriate implant size and length. Because it is difficult to achieve the goal perfectly with the traditional surgical guide, the CAD/CAM surgical guide is useful for virtual implant planning using radiographic data and dental cast models. In the present case, clear CT images were difficult to obtain and the advantage of guided surgery could not be applied because of the patient’s postural instability.
Histological evidence indicates that 84% of patients with CKD have bone disorders.5 Regarding renal osteodystrophy, bone demineralization, decreased trabeculation, and abnormal bone healing after tooth extraction may occur. Since bone structural integrity is likely to change in patients with CKD, it is important to secure implant stability at various points in time and predict the long-term prognosis based on the measured stability. In patients with CKD, considering the high risk of infection and delayed hemostasis during dental treatment, the number of surgeries and immediate implantation using a flapless surgical approach should be considered. The advantages of implant placement immediately after tooth extraction are that it can minimize the loss of soft and hard tissues by shortening the treatment period, reducing the number of surgeries, and allowing a flapless surgical approach. In addition, the extraction fossa based on the original tooth could be a reference point and the implant could be placed in an ideal three-dimensional position. However, contrary to the previous opinion that the loss of surrounding tissues is prevented, the possibility that resorption of the buccal alveolar bone may proceed further has been suggested when implant placement is performed immediately after tooth extraction.6 In patients with periodontal disease, many alveolar bones, including the buccal cortical bone, are absorbed, and there is a risk of immediate implant failure owing to soft tissue recession. Delayed implantation is recommended in these cases; however, an effort could be made to compensate for bone loss by performing extraction in a minimally invasive way to minimize trauma to the surrounding alveolar bone and graft a bone material that takes a long time to absorb on the buccal side. In the present case, a 1:1 mixture of allogeneic bone graft material with excellent bone quality induction ability and xenogenic bone graft material that required a long time to absorb was transplanted into the gap created during immediate implantation, resulting in compensation for bone loss.
In general, immediate loading is applied within 1 week after implant placement, while conventional loading is implemented within 3–6 months after implant placement. Early loading refers to the application of a load at a time point between those for immediate and standard loading.7 Because implant treatment requires a long time in partially or completely edentulous patients, an immediate load relieves this discomfort. Thus, many cases of immediate loading have been reported; however, there remains no consensus regarding the timing of implant loading.
As most studies on immediate loading describe an insertion torque ≥30 Ncm, case selection is important.8 In the present case, after implant replacement, conventional loading was determined using a one-piece temporary bridge. For each of the eight implants placed in the maxilla and mandible, the insertion torque was <30 Ncm, except for implants #17i, #26i, #27i, #37i, and #46i. To ensure the implant stability, the fixation was confirmed to be well maintained by measuring an index with a fixation measurement instrument (Mentor, Osstell® ISQ) (Tables 1 and 2). Conventional loading was performed after 3–4 months using a temporary bridge. A previous study investigated the factors affecting implants and prostheses survival, failure, and complications according to the implant loading protocol.9 Moreover, few reports have assessed the success rate of immediate loading for implant placement in patients with CKD. Therefore, compared to other patients, it is crucial to observe the sequence of RFA values in patients with CKD.
DM is the aggregate of all metabolic diseases with high blood sugar levels. DM is the leading cause of ESRD, non-traumatic lower-extremity amputation, and blindness in adults, and the prevalence of DM is increasing worldwide. Therefore, the precautions for implant treatment in patients with diabetes must be understood. Hyperglycemia causes excessive oxidative stress, which impairs angiogenesis and reduces bone formation.10 In particular, patients with hemoglobin A1c (HbA1c) levels >10% have increased levels of advanced glycation end products (AGEs) in the peri-implant sulcular fluid.11 Studies comparing implant failure rates between diabetic and non-diabetic patients reported that the implant failure rate was not higher in diabetic patients with normal or near-normal glucose levels compared to those in the normal population at the 1-year follow-up after implantation.12 Although peri-implant bleeding increased as HbA1c levels increased, 57 of 59 implants showed a high survival rate of 96.6% after 2 years of implant treatment in the follow-up of patients with poorly controlled type 2 DM (8.0% ≤ HbA1c ≤ 12.0%).13 In most of the literature, patients with diabetes with well-controlled implant survival did not differ significantly from those without diabetes; however, the bone level loss was significant in patients with diabetes regarding changes in marginal bone levels around the implants.14
DM is a chronic disease characterized by impaired glucose, lipid, and protein metabolism due to defects in insulin action or secretion. These metabolic disorders cause chronic diabetic polyneuropathy, and CKD exacerbates diabetic polyneuropathy.15 In patients with diabetic neuropathy, it may be difficult to obtain a clear CT image owing to an unstable posture. Guided surgery increases the accuracy of flapless implant placement. However, intraoperative accuracy requires perfect fitment and safe reproducibility. The traditional cast-based surgical guide may be helpful for patients who have difficulty undergoing CT scans. The three main methods of creating a cast-based surgical guide are (1) diagnostic wax-up, (2) denture tooth setup, and (3) duplication of pre-existing teeth or restorations. Generally, cast-based surgical guides only indicate the proper position of the crown to be restored and do not consider bone morphology. Therefore, special care must be taken when cast-based surgical implant guides are used. In this patient, radiographs were obtained with a prefabricated guide to confirm the vertical bone height and direction of implant placement (Fig. 6). Because the bone morphology was not considered in the surgical guide, the implant location on the buccal-palatal side was determined with flap elevation during the surgery (Fig. 7C).
Ⅳ. Conclusion
The present case received a proper treatment plan with a conventional surgical guide and implant placement in a patient with CKD undergoing hemodialysis who was completely edentulous after tooth extraction due to severe caries and periodontal disease.
Given the patient’s medical condition, primary stability of the implant and conventional rather than immediate loading were important. Control of the antibiotic dosage and selection of an appropriate analgesic are needed due to the risk of drug toxicity in patients with impaired renal function. Drug discontinuation, change, or maintenance must be considered in patients receiving antithrombotic therapy. While placement immediately after tooth extraction is a well-established surgical method and has the advantage of reducing the number of surgeries, it can complicate the surgery due to bone gaps and defects.
Thus, patients on dialysis or with DM can achieve successful treatment results by considering the dialysis treatment date, drug dose adjustment, and laboratory values. Even with these considerations, minimally invasive surgery is recommended in these patients.