Ⅰ. Introduction
As implants become a predictable treatment option to replace the edentulous area, more attention is paid to shortening the treatment period and adjusting the treatment process to the convenience of the operator and the patient.
In the case of tooth extraction due to severe loss of alveolar bone, ridge preservation can be performed simultaneously with extraction, minimizing tissue absorption and maximizing bone formation, thus reducing the healing period for subsequent treatments such as implantation.1 Ridge preservation can be applied in various combinations of bone materials and membranes or biomaterials such as platelet-rich plasma (PRP) and platelet-rich fibrin (PRF). Applying autologous platelet concentrates to the extraction site promotes epithelialization and initial healing of the soft tissue, reducing postoperative discomfort and pain.2 PRF can be manipulated into a dense fibrin-rich matrix, which, compared to PRP, makes it less complicated to fabricate, simpler to prepare, and easier to manipulate in the application and sealing process.3 A report shows that using a bone graft material together with PRF is more beneficial than using only a bone graft material when performing ridge preservation procedures.4 The extraction site is an open wound in nature; however, applying the PRF in the form of a membrane and covering it on the top of the bone graft material helps stabilize the bone material in position within the extraction socket and improve the stability of the wound for vascularization.5
If ridge preservation is performed, the bone regeneration process of the applied material can be achieved throughout the healing period between extraction and implantation, thus shortening the entire treatment period.6 When placing the implant after healing, the guided implant surgery method helps place the implant in a more accurate position, as the bone tissue of the extraction socket is not completely mineralized. Poor bone quality usually makes it difficult to accurately drill to the desired position during the high-speed drilling process; however, in guide implant surgery, it is possible to place the implant in the “prosthetic-driven” position planned by the operator using a pre-manufactured surgical guide with low-speed drilling.7 Furthermore, guided implant surgery can prevent bone loss due to flap elevation, as the flapless method can prevent the exposure of new bone tissue that will be mineralized after the ridge preservation procedure. Furthermore, patient discomfort, such as postoperative edema, pain, and hemostatic problems, has decreased compared to conventional implant surgery methods.8
Herein, we report the long-term prognosis (>6 years) in two cases where the alveolar bone loss was severe at the extraction site, ridge preservation was performed using xenografts and PRF membranes at the time of extraction and the implants were placed using guided implant surgery.
Ⅱ. Case Report
1. Case 1
A 53-year-old male patient complained of discomfort during a bite of the mandibular right second molar. During clinical examination, the tooth showed mobility grade 3, and pathological bone resorption that progressed beyond the root apex of #47 was observed on radiography (Fig. 1A, 1B). Consequently, the tooth was diagnosed as hopeless and an extraction was planned. The patient was a heavy smoker who smoked one pack of cigarettes daily. Before tooth extraction, smoking cessation education was provided, and nonsurgical periodontal treatment was performed on all dentitions. As the tooth was extracted, the prepared PRF membrane and xenograft material (Bio-Oss; Geistlich, Wolhusen, Switzerland) were used to perform alveolar ridge preservation simultaneously (Fig. 1C). The PRF membranes were prepared using the following process. Blood was taken (approximately 10 ml), placed in tubes without anticoagulant and immediately centrifuged at 3000 rpm for 12 min. Surgical tweezers were then used to remove the whitish and yellowish product of centrifugation, leaving behind the acellular supernatant (platelet-poor plasma, PPP) in the tube. Finally, the PRF layer was separated from the lower layer, which is rich in red blood cells, using scissors. The PRF was then compressed between wet sterile gauze to form a membrane (Fig. 2A). The tooth extraction was completed, and the internal granulation tissue was thoroughly removed until the sound bone was exposed. The bone graft material was then applied to the subgingival level within the extraction site, and the coronal part was covered with a PRF membrane beneath the gingiva and sutured using a figure-of-8 suture method (Fig. 2B to 2D). During extraction, extensive bone loss occurred on the buccal, mesial, and distal sides. According to the classification proposed by Kim et al.,9 the extraction site was classified as type V (alveolar bone loss >50% at the buccal or lingual site with gingival recession). The healing process was uneventful and implant placement was planned approximately 3 months after the gingival tissue was determined to have healed appropriately. After setting the ideal implant placement position by scanning the surgical site using an oral scanner (TRIOS; 3Shape, Copenhagen, Denmark), a surgical guide was fabricated (Fig. 1D, 1E). The surgery was performed according to the manufacturer’s protocol (Dio, Busan, Korea), and an implant with 4.5 mm in diameter and 10 mm in length was inserted with an initial torque of 40 N (Fig. 1F). The final prosthesis was delivered approximately 3 months after implantation (Fig. 1G) and had been functioning well after approximately 6 years and 4 months, and it can be observed that the bone density of the alveolar bone crest has increased over time (Fig. 1H to 1J).
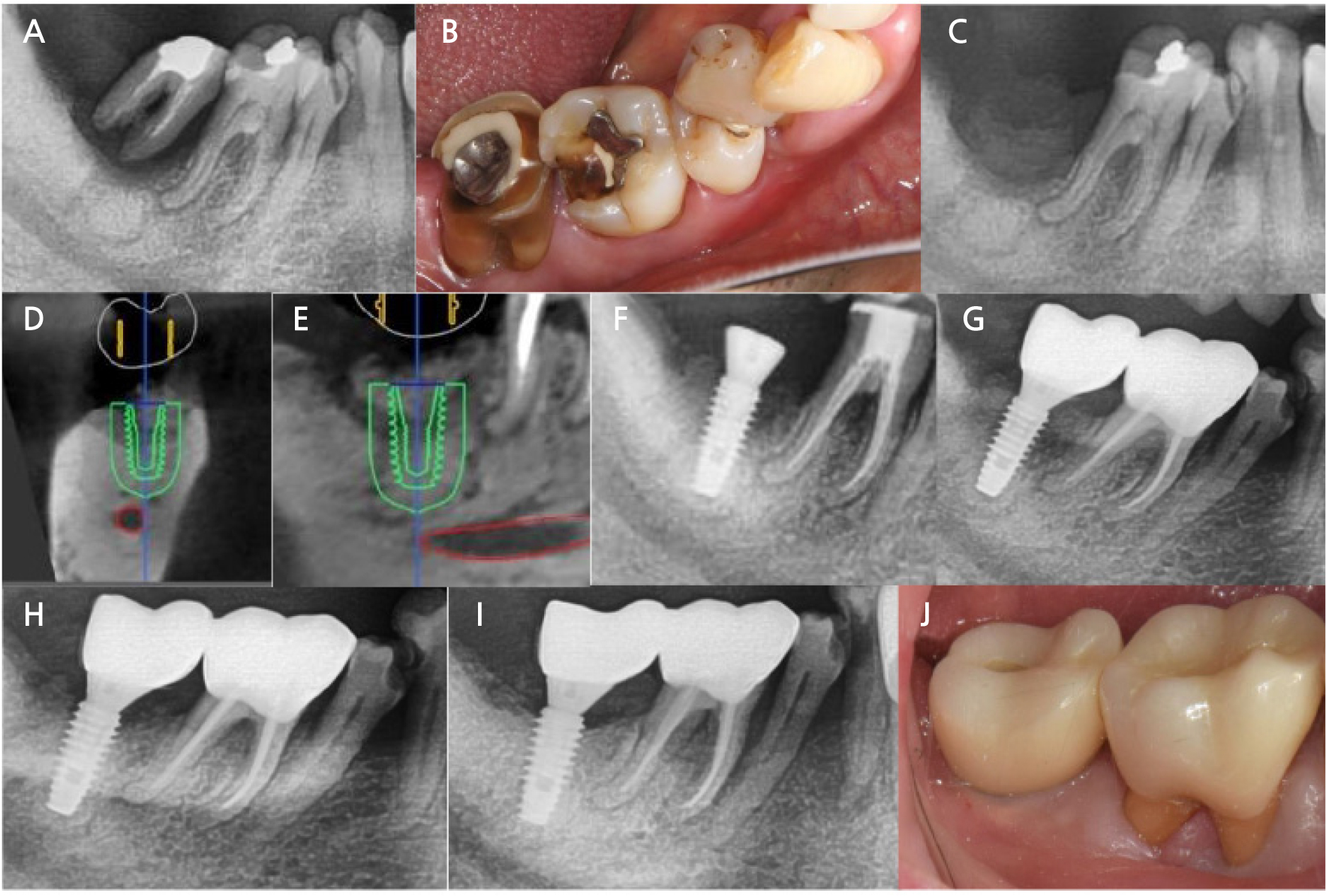
Fig. 1.
Clinical and radiographic view of Case 1. (A and B) Initial clinical and radiographic view, (C) Radiographic view after extraction and preservation of the socket, (D and E) Pre-surgical treatment planning using a computer program, (F) Radiographic view after implant placement, (G) Radiographic view after prosthesis setting, (H) Radiographic view after one year of prosthesis setting, (I and J) Radiographic and clinical view after 6 years and 4 months.
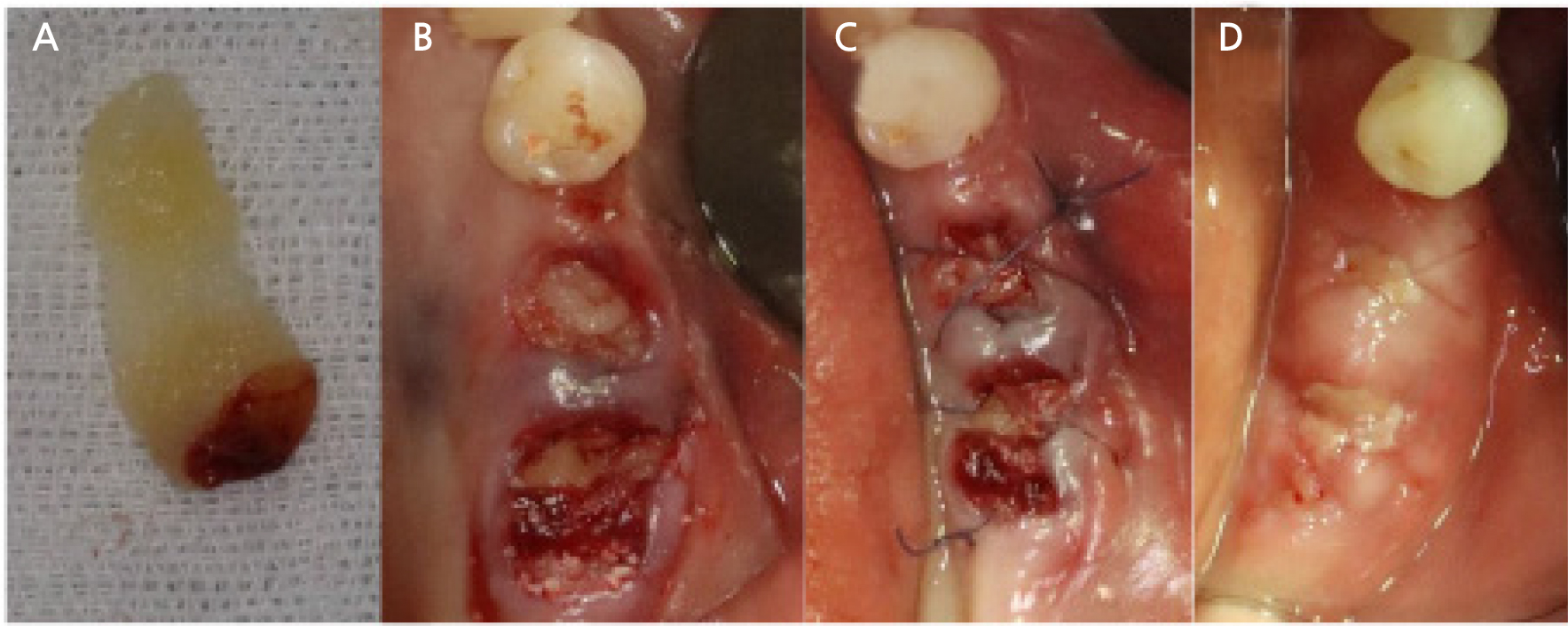
Fig. 2.
Representative clinical view of the ridge preservation procedure used in these cases. (A) Platelet-rich fibrin membrane made by centrifuging the collected blood and compressing it, (B) Clinical view of filling the extraction socket with xenograft material and covering with PRF membrane, (C) Suture, (D) Stitch-out after 1 week.
2. Case 2
A 37-year-old female visited the clinic with the chief complaint that her tooth (#47) had fallen out due to severe mobility the day before her visit (Fig. 3A). The panoramic view showed a severe loss of alveolar bone at the extraction site, invading the distal root of #46. When the extraction site was naturally healed, it was expected that the alveolar bone in the area would be severely atrophied after healing; therefore, we decided to perform ridge preservation (Fig. 3B). The extraction site was thoroughly debrided, as observed on the radiograph, and we visually confirmed that the residual buccal bone of the extraction socket was less than half the root of the adjacent tooth and that the distal root of #46 was not apically involved. According to the classification proposed by Kim et al.,9 this case was type IV (alveolar bone loss >50% at buccal or lingual site without gingival recession). The xenograft material (Bio-Oss) was inserted up to the adjacent bone level, and then the PRF prepared from the patient's venous blood was compressed and laid over the bone material and sutured (Fig. 2). Healing was uneventful; tooth #46, which had affected the apex of the distal root at the first visit, showed a normal response on the electric vitality test with no clinical symptoms; therefore, we decided to maintain the tooth. The soft tissue at the extraction site had healed in an adequate shape approximately 3 months after preservation of the ridge, and implant surgery was planned. The surgical site was scanned, CT was performed, and we planned to install a 4.5 × 8.5 mm implant at the extraction site (Fig. 3C to 3E). Surgery was performed according to the manufacturer’s protocol (Dio, Busan, Korea) (Fig. 3F). An implant of the planned size was placed at the planned location with an insertion torque of 40 N (Fig. 3G, 3H). Approximately 3 months after implant placement, the final prosthesis was delivered. Radiography confirmed that the alveolar bone around the implant was stable, and the distal apex of #46 also showed a normal periodontal ligament space (Fig. 3I, 3J). Currently, 6 years and 8 months have passed since prosthesis delivery, and the implant is functioning stably without loss of the crestal alveolar bone (Fig. 3K, 3L). Additionally, it can be confirmed that the alveolar bone at the distal apex of #46 is maintained stably.
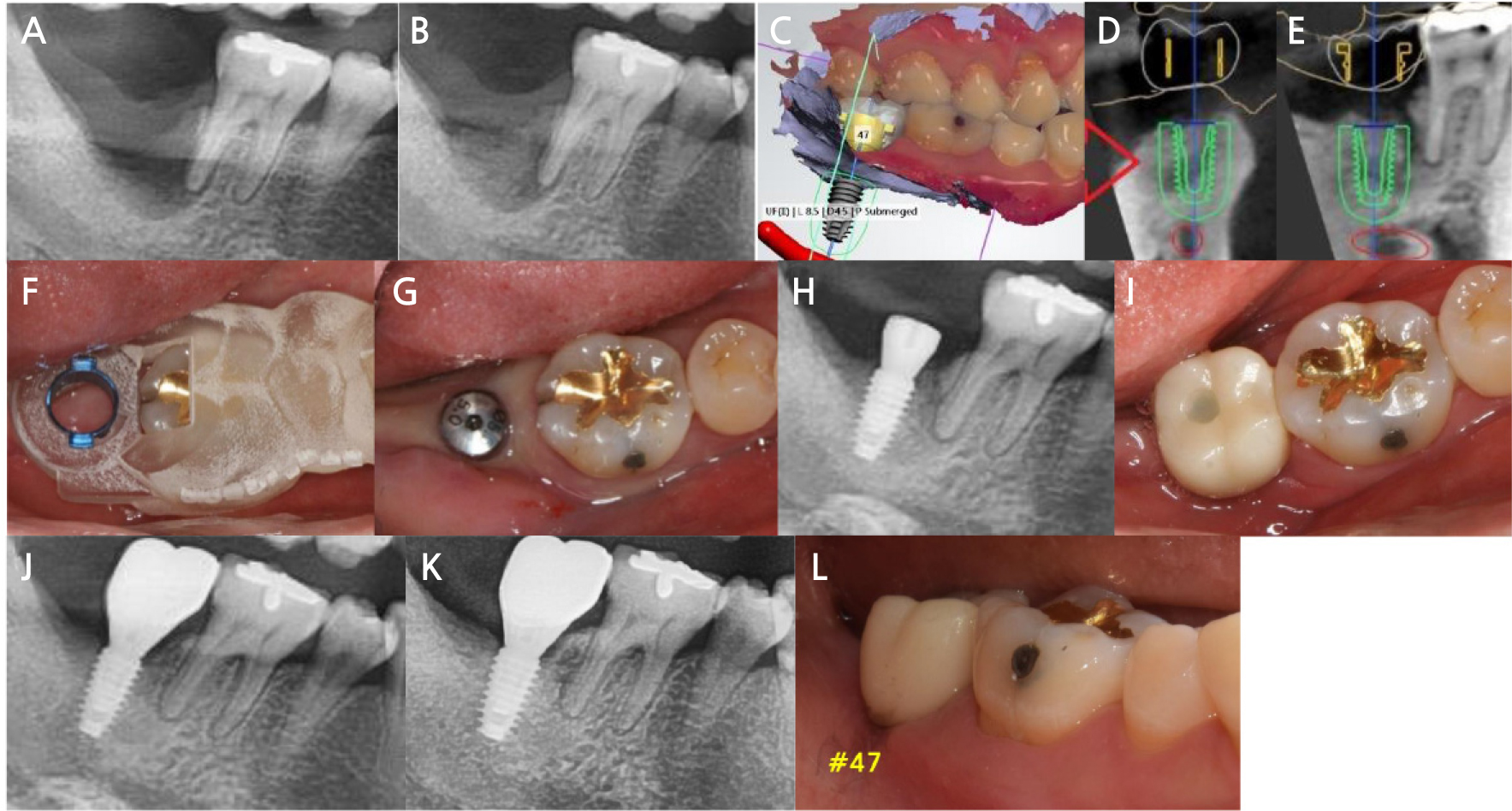
Fig. 3.
Clinical and radiographic view of Case 2. (A) Initial radiographic view, (B) Radiographic view after extraction and preservation of the socket, (C-E) Planning of pre-surgical treatment using a computer program, (F) Clinical view with surgical guide, (G and H) Clinical and radiographic view after implant placement, (I and J) Clinical and radiographic view after the setting of the prosthesis, (K and L) Radiographic and clinical view after 6 years and 8 months.
Ⅲ. Discussion
The timing of implant placement is determined according to the dimension of partial or complete healing of soft and hard tissues and can be traditionally classified as early implant placement (4−8 weeks after extraction), delayed implant placement (3−4 months after extraction), or late implant placement (more than 4 months after extraction).10 Early implant placement is usually performed between 4 and 8 weeks after extraction, and it is considered that the soft tissue has healed enough to allow proper flap management during surgery.11 However, since the time is short for the formation of new bone in the extraction socket, sufficient bone quality cannot be guaranteed at this time. In contrast, late implant placement provides a sufficient healing period of ≥4 months after tooth extraction, allowing bone growth at the extraction site. However, bone resorption that inevitably follows tooth extraction cannot be prevented while the treatment period is still prolonged.12 According to the consensus of the European Workshop on Periodontology (2018) on the treatment of tooth extraction and implant placement, when ridge preservation was performed with tooth extraction, the implant placement time was defined as a modified form of delayed or late placement.13 When ridge preservation is performed, compared with tooth extraction alone, bone resorption can be prevented by 1.5−2.4 mm horizontally and 1−2.5 mm vertically. Subsequently, bone graft material and PRF membrane were used together to preserve the ridges of the sockets with severe bone loss in our cases, and the implants were installed after 3 months of healing period to reduce the complexity of guided bone regeneration procedures and solve the problem of delayed healing.
Araujo et al.14 examined changes in the alveolar ridge by placing a bone graft material in the socket at the time of extraction and covering it with an autologous soft tissue graft and observed that the dimensional change of the hard tissue after extraction was minimized. Our case involved the use of PRF membranes to cover bone graft materials, in which growth factors help maximize the initial healing process. Growth factors, such as platelet-derived growth factor, tumor growth factor-ß1, and vascular endothelial growth factor, are contained in the fibrin matrix of PRF and are released slowly (approximately 7 days) after application, contributing to cell growth and proliferation.15 After tooth extraction, it is known that approximately 8−12 weeks are needed for the dense connective tissue to fill the socket and undergo bone maturation. However, when PRF is applied to the extraction socket, trabecular bone can be observed in approximately 30% of the new tissue after 6 weeks.6 In the procedure for bone regeneration at the extraction site or bone defect site, covering the coronal part of the bone graft material with the PRF membrane is said to promote regeneration of the alveolar bone and gingiva by stimulating differentiation and proliferation of epithelial cells, gingival fibroblasts, osteoblasts, and mesenchymal stem cells.5 According to an in vitro study comparing the PRF membrane and absorbable collagen membrane as a scaffold for periosteal tissue engineering, the PRF membrane was more effective in the proliferation of periosteal cells than the collagen membrane.16
Because PRF membranes can be easily prepared using autologous blood, they have advantages in cost, convenience, and operability. Furthermore, since it does not require primary closure of the extraction site, it can be assessed by a flapless or minimally invasive approach. As it is an autologous material, complications, such as infection related to membrane exposure, are minimized. Because there was no change in flap position, there was no excessive displacement of the mucogingival junction. Therefore, the difficulty of the procedure is not high, providing convenience for the operator and the patient.17 In our cases, by performing ridge preservation using the PRF membrane on the day of tooth extraction, it was possible to satisfy the needs of patients who wanted to shorten the treatment period. Furthermore, it accelerated healing and avoided extensive bone grafting during subsequent implant placement, thus lowering the sensitivity of the procedure, which is an operator-dependent factor.
In our cases, the advantages were maximized using the guided surgery method for implant placement. Most of the current guide implant surgery methods follow the flapless method, which is performed without incision and elevation of the flap and maintains the blood supply of soft tissues that can affect gingiva healing.18 Exposure of alveolar bone during surgery is inevitably accompanied by bone loss; therefore, when an implant is installed flapless, the marginal bone around the implant can be more protected than the flap elevation method. According to the flapless method, the periosteum can be completely preserved during the operation, allowing the supraperiosteal plexus to remain above the periosteum and blood supply, maximizing the osteogenic potential.19 In these cases, since implant placement was performed after ridge preservation using the PRF membrane, we used a flapless guided surgery technique to minimize bone exposure and maintain bone formation at implant sites as much as possible. Additionally, implants using the guided surgery technique could be placed in an ideal location regardless of the bone quality of the crestal part, which was relatively less healed. In particular, as in our cases, in which bone resorption was severe, for implant placement 3 months after the ridge preservation procedure, it was difficult to place the implant in an accurate position and obtain sufficient final insertion torque due to the quality of the immature bone. However, because guided implant surgery has an error range of approximately 1.09 mm and 1.28 mm in the crestal and apical region, respectively, it is more advantageous to position the implant in the “prosthetic-driven position” compared to conventional surgery.20
Ⅳ. Conclusion
In these cases, where severe alveolar bone resorption was observed after tooth extraction, ridge preservation was performed using bone graft material and PRF membrane simultaneously to induce rapid healing and maximize the healing potential for implant placement. Subsequently, using the guided implant surgery method, the implant could be placed in the ideal position intended by the operator with sufficient torque. Therefore, the overall treatment period could be shortened, and the difficulty of the procedure could be reduced, resulting in satisfactory treatment results for the patient and the operator. The implants were stable for over 6 years without loss of marginal bone in both cases.




