Ⅰ. Introduction
In cases of common alveolar bone deficiency in the posterior edentulous maxilla, dental implant treatment can proceed following reconstruction of the defect through alveolar ridge augmentation and/or maxillary sinus floor augmentation (MSFA), depending on the specific characteristics of the bone deficiency. Guided bone regeneration and MSFA via a crestal or lateral approach are well-established surgical techniques for bone regeneration in the posterior edentulous maxilla in the field of dental implant.1,2 However, in cases involving extensive alveolar defects with large sinus floor perforations caused by severe periodontitis, osteonecrosis, trauma, or complications from prior treatments, such as post-MSFA infection or tumor resection, reconstruction remains challenging.
Various types of bone substitutes with distinct characteristics are used in dentistry. However, most of these materials, except autografts, autogenous tooth bone grafts, and certain allografts, are only osteoconductive.2 Consequently, they tend to undergo resorption during the bone regeneration process, resulting in a reduction in volume compared to the initial grafted amount, which is often difficult to predict.3 In the development of new bone substitutes, growth factors, particularly bone morphogenetic protein-2 (BMP-2), have gained attention owing to their potent osteoinductive properties and are already commercially available.4 BMP-2 has shown exceptional osteoinductive capabilities in numerous animal studies and off-label clinical trials.5,6Despite the United States Food and Drug Administration's approval of BMP-2 for maxillofacial applications in 2007,2,7 its clinical efficacy remains disputed with limited substantiating studies.2,3,8,9,10,11 In addition, limited cost-effectiveness and lack of familiarity restrict its widespread use in clinical practice.8
Although the outcomes vary depending on the type of carrier used, our previous studies have shown that BMP-2 yields superior results in terms of density and volume stability compared to conventional bone graft materials.2,3,8 Furthermore, we successfully managed extensive posterior maxillary bone defects using recombinant human BMP-2 (rhBMP-2) with hydroxyapatite (HA) and fibrin sealant (FS) as carriers. This report presents two cases that demonstrate the efficacy of this approach.
Ⅱ. Case Report
1. Case 1
A 42-year-old woman with no systemic disease was referred to our hospital following the unsuccessful management of sinusitis due to an infection after MSFA at the right upper first molar (#16) site, which had been performed at a private dental clinic. Upon presentation, an oroantral fistula (OAF) with purulent discharge was observed. The adjacent second molar (#17) was severely mobile with mesial inflammation. The initial treatment involved the administration of antibiotics, extraction of #17, and curettage of the contaminated graft material intraorally, followed by the closure of the OAF using a buccal mucosal advancement flap. After significant soft tissue healing and regeneration of the sinus membrane, alveolar ridge reconstruction was performed. Preoperative computed tomography (CT) revealed a large perforation in the posterior maxilla and more than half of the buccal alveolar ridge was compromised (Fig. 1). A palatal incision was made (Fig. 2A), the mucoperiosteal flap was elevated using a molt surgical curette, and the sinus membrane was carefully detached from the gingiva. This revealed a large bone defect with a well-healed internal sinus membrane (Fig. 2B). The sinus membrane was gently elevated through the bone perforation site using a sinus curette. No additional bone reduction was performed. Due to the extensive bone defect, 0.5 g of biphasic calcium phosphate (BCP, Frabone-I; Inobone, Cheonan-si, South Korea) was placed in the sinus medial and distal areas to fill the space, and a single chunk of rhBMP-2/HA (Novosis-Dent; CG Bio, Gyeonggi-do, South Korea) 1.0 g combined with fibrinogen/factor XIII and thrombin FS (Greenplast Q; GC Medical Science Corp., Chungcheongbuk-do, South Korea) 2 ml was grafted into the defect to reconstruct the alveolar ridge (Figs. 1B and 2C). Tension-free suturing was performed after covering the graft with a collagen membrane (Jason Membrane; Botiss Biomaterials, Zossen, Germany). At the 6-month postoperative follow-up, CT imaging demonstrated that the reconstructed alveolar ridge maintained a stable shape with increased radiopacity (Fig. 1C). Using an analytical method from previous studies to measure volume changes within a specific density range of 200–2000 Hounsfield units, a 28.48% increase in volume was observed compared to that observed immediately after the surgery. Dental implants were subsequently placed at sites #16 and #17 without additional bone grafting (Fig. 2D). The final insertion torque for both implants was 50 Ncm. One month after placement, the implant stability value (ISV), measured using an implant stability measuring device (The Trust; Dentium, Seoul, South Korea), was 69 for #17 and 70 for #16. Three months after placement, the ISV increased to 70 for #17 and 84 for #16, indicating stable integration (Fig. 2E). Subsequently, prosthetic implant restorations were performed. At the 30-month follow-up after implant restoration, the reconstructed alveolar ridge showed stable maintenance, with increased radiopacity (Fig. 1D).
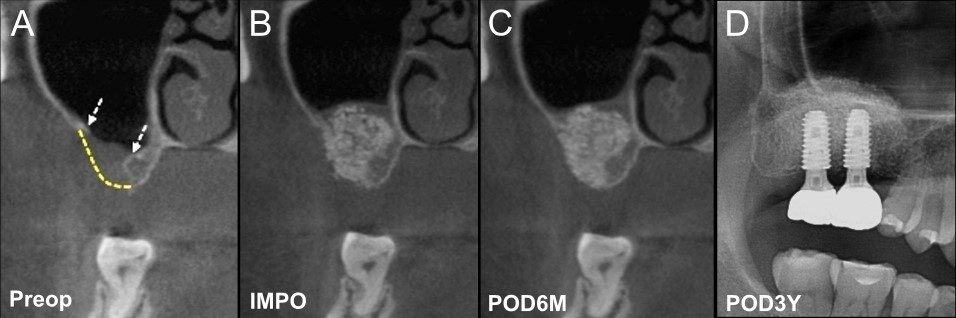
Fig. 1.
Pre- and post-operative radiographs of Case 1. (A) Coronal computed tomography (CT) view of pre-operation (Preop). White dotted arrows indicate the boundaries of the bone defect, and the yellow dotted line represents the presumed original contour of the alveolar ridge, (B) Coronal CT view immediate post-operation (IMPO). The bone graft material is placed as a single piece to reconstruct the extensive alveolar ridge defect, (C) Coronal CT view of 6 months post-operation (POD6M). It demonstrates an increase in density while maintaining its original shape compared to IMPO, (D) Radiograph at 3 years post-operation (POD3Y). It shows stable maintenance of the grafted materials and dental implants.
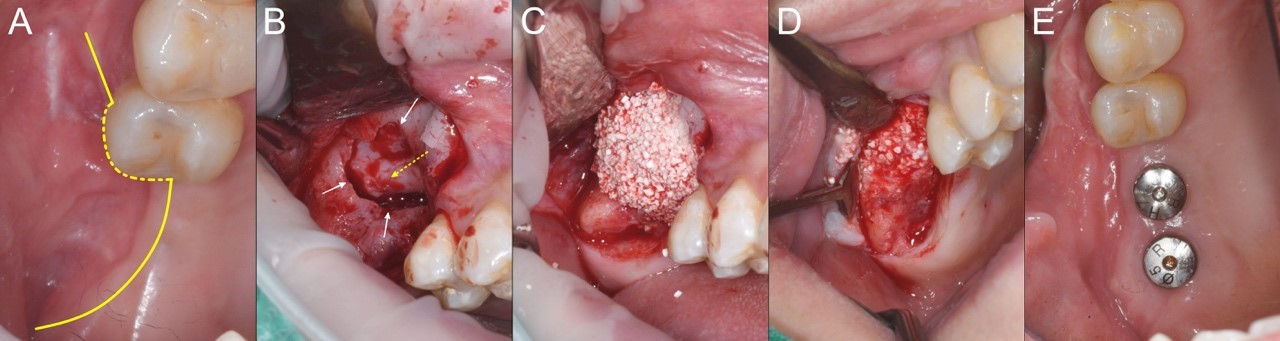
Fig. 2.
Surgical procedure in Case 1. (A) Occlusal view of the operation site showing severe buccal recession of the alveolus. The yellow line indicates the incision line, (B) A large bone defect (white arrows) is visible after gingival elevation, with careful separation of the adherent gingiva and Schneiderian membrane (yellow dotted arrow), (C) A single chunk of BMP-2/HA/FS is grafted into the defect to reconstruct the alveolar ridge, (D) Healing status at 6 months post-operation, showing that the carrier particles remain and appear to be tightly bound, (E) Status 3 months after implant placement, demonstrating a well-restored alveolar bone morphology.
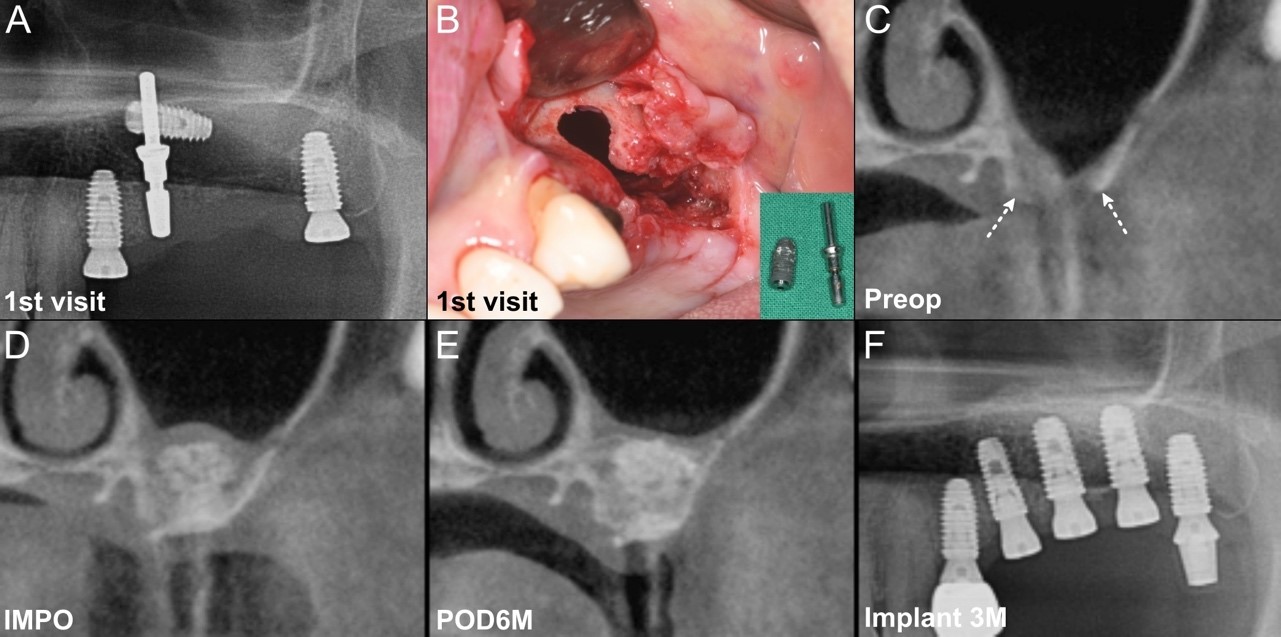
Fig. 3.
Surgical progress in Case 2. (A) Radiograph at the first visit shows an implant fixture and a guide pin within the left maxillary sinus, displaced during implant placement at a local dental clinic, (B) The gingiva is elevated, and the preexisting perforation is further widened to remove the foreign materials, (C) Coronal computed tomography (CT) view of pre-operation (Preop). White dotted arrows indicate the bone defect boundaries, (D) Coronal CT view of immediate post-operation (IMPO), (E) Coronal CT view of 6 months post-operation (POD6M) showed increased density and volume compared to IMPO, (F) Radiograph at 3 months post-implant placement.
2. Case 2
A 59-year-old woman with no significant medical history was referred to our hospital after an iatrogenic accident that occurred during implant placement at a private dental clinic. Panoramic radiography revealed an implant fixture and guide pin inside the left maxillary sinus (Fig. 3A). Upon raising the gingiva to remove the foreign bodies, it was observed that the referring dentist had already removed a significant portion of the alveolar ridge in an attempt to retrieve it, resulting in a considerable defect in the bone (Fig. 3B). After the careful removal of the foreign bodies from the maxillary sinus, tension-free suturing was performed to close the OAF. Following a 3-month healing period, alveolar ridge reconstruction was performed similarly as described in Case 1 (Fig. 3C and 3D). A total of 1.0 g of BMP-2/HA with 2 ml of FS covered with a collagen membrane was used without adding a BCP graft. At the 6-month follow-up, radiographs showed increased radiopacity and satisfactory healing of the reconstructed site (Fig. 3E). Implant placement was successfully performed in the upper left second premolar (#25), first molar (#26), and second molar (#27). The final insertion torque for all three implants was > 50 Ncm. One month after placement, the ISV were 76 for #27, 81 for #26, and 77 for #25. Three months after placement, the ISV of all three implants increased to 84, indicating stable integration (Fig. 3F). Implant prosthetic restoration was completed at a local dental clinic.
Ⅲ. Discussion
Although procedures for alveolar bone augmentation, such as guided bone regeneration and MSFA, are well established, the reconstruction of large maxillary bone defects, including extensive sinus floor perforations, remains challenging. The unpredictable degree of bone resorption and difficulties in soft-tissue healing, which are often exacerbated by the use of a metal mesh barrier for space maintenance, contribute to these challenges. The technique of alveolar bone reconstruction using BMP-2/HA/FS has been applied to numerous MSFA cases and has demonstrated superior results compared with conventional bone graft materials.3,8 In a previous study on MSFA, BMP-2/HA showed a graft volume change of 26.44% ± 7.78% over 6 months, compared to –2.92% ± 30.92% for conventional bone substitutes, indicating a statistically significant difference.3 Another study comparing BMP-2/HA with BMP-2/HA/FS in MSFA found graft volume changes of 29.77% ± 13.42% and 48.75% ± 37.44% over 6 months, respectively, also indicating a statistically significant difference.8 The use of FS with BMP-2/HA resulted in better outcomes than the use of HA alone, suggesting that FS may act as an effective supplementary carrier of BMP-2 by enhancing its localization and controlled release. This effect was also demonstrated in a previous animal study using FS as a BMP-2 carrier.5 Additionally, FS improves graft maneuverability, making the procedure more efficient and reducing the operative time. It also prevents bone graft displacement even if the Schneiderian membrane is perforated during elevation.8 Furthermore, the use of FS offers several advantages in reconstructing extensive bone defects as it provides shape retention and material fixation without the need for additional scaffolds.
Some clinical studies have not shown significant superiority of BMP-2 over conventional bone substitutes, particularly MSFA.12,13 However, these studies used an absorbable collagen sponge or BCP as carriers for BMP-2, and differences in carriers may account for the varied results.3 The delivery system controls the localization and release of growth factors, maintains space for new bone formation, and plays a crucial role in determining their efficacy.14 Compared to ACS and BCP, HA may offer advantages as a BMP carrier by better maintaining the space for new bone formation.2,3 Additionally, advanced forms of HA, such as HA nanoparticles and spherical porous HA microparticles, can provide a sustained-release profile when used as protein carrier.15,16
Nevertheless, the use of BMP-2 has not been explored by many clinicians. Furthermore, it is often a concern that complications such as swelling may occur following bone grafting with BMPs. However, based on previous studies and the author’s experience, including these cases, no notable complications were observed. The aurthor believes that predictable and reliable bone grafting can be achieved with reduced amounts of bone graft material when BMP-2 is paired with appropriate carriers.2,3,8 These cases demonstrate that effective bone reconstruction and implant placement are feasible even in challenging posterior maxillary bone defects that are typically difficult to manage with conventional grafting materials, ultimately restoring oral function.




