Ⅰ. Introduction
Following tooth extraction, bone remodeling frequently results in insufficient ridge dimensions for optimal implant placement.1 This issue is particularly pronounced in the posterior maxilla, where maxillary sinus pneumatization often exacerbates bone deficiency. Techniques, such as lateral approach sinus floor elevation (LSFE) and guided bone regeneration (GBR), have been developed to address this challenge. The predictability and safety of both techniques have been extensively documented.2,3 Studies have shown that combining LSFE and GBR in edentulous patients yields high mid-term implant survival rates.4
Various materials have been studied in the fields of GBR and LSFE. Among these, two commonly discussed biomaterials are autologous bone (AB) and anorganic bovine bone mineral (ABBM), which demonstrate comparable implant survival rates and outcomes similar to those of implants placed in pristine bone.5 However, their histological properties differ significantly.6ABBM alone offers effective space maintenance but may exhibit reduced vital bone formation because of its slow turnover rate, resulting in a higher proportion of residual graft material. Conversely, studies have indicated that combining ABBM with at least 40% AB leads to significantly greater bone gain, attributable to the osteogenic potential of AB.7Nonetheless, the use of AB alone is discouraged because of potential resorption issues.8 Moreover, AB employment is constrained by limited bone volume, surgical complications, and postoperative morbidity. Consequently, synthetic and biomimetic bone substitutes are potential alternatives to AB.
Recently, octacalcium phosphate (OCP, Ca8H2[PO4]6 5H2O) has emerged as a novel synthetic bone graft material.9 OCP, serving as a direct precursor of biological apatite, has emerged as a novel synthetic bone graft material and has demonstrated effectiveness in promoting new bone formation because of its rapid biodegradability and strong osteogenic potential.10 In our previous pilot study, we assessed the effectiveness and feasibility of bone regeneration in LSFE using biomimetic OCP synthetic bone substitute material through radiographic and histomorphometric evaluation.11
This case report describes the use of ABBM in conjunction with biomimetic OCP for the rehabilitation of a severely atrophic edentulous maxilla.
Ⅱ. Case Report
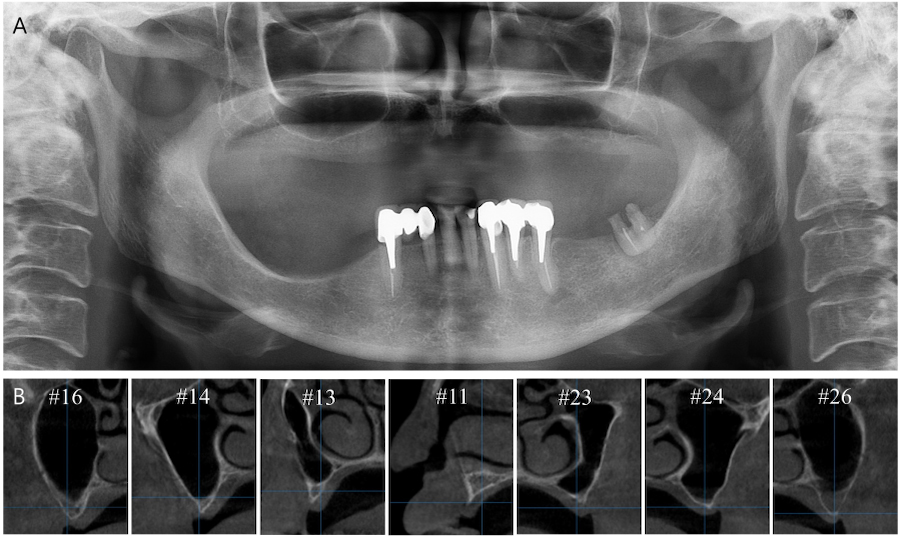
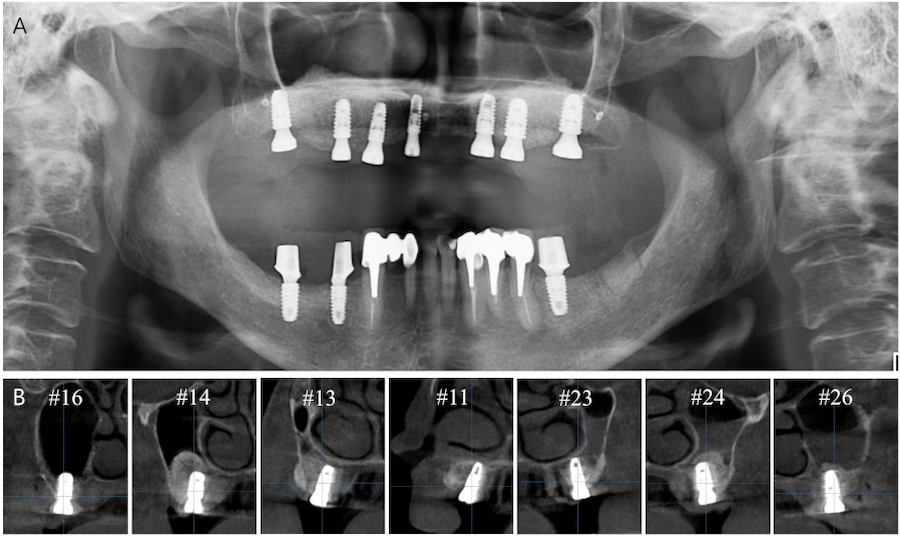
A 62-year-old woman with no relevant medical history visited Chosun University Dental Hospital in 2021. The patient’s main concern was rehabilitation of the edentulous maxilla. Cone-beam computed tomography (CBCT) revealed insufficient alveolar bone height and width for implant placement (Fig. 1). After thorough evaluation, vertical and horizontal GBR was planned for the severely atrophic maxilla, including bilateral LSFE.
1. Surgical procedures
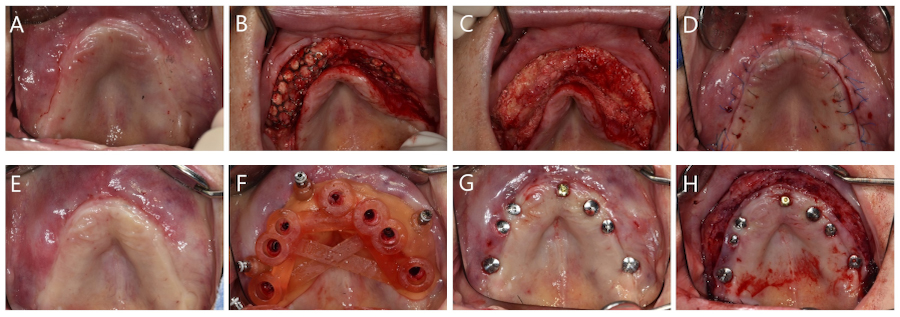
All surgical procedures were performed by a single skilled periodontologist (WPL). Under local anesthesia, a full-thickness mid-crestal incision was made into the keratinized mucosa using a #15 scalpel. A full-thickness flap was elevated to expose the lateral walls of both maxillary sinuses (Fig. 2A and 2B). After preparing the bony window using a piezoelectric device (Fig. 2C), the Schneiderian membrane was carefully elevated (Fig. 2D). ABBM (Bio-oss®; Geistlich Pharma AG, Wolhusen, Switzerland) and biomimetic OCP synthetic bone substitute material (Bontree®; HudensBio Co., Gwangju, Korea) were used in a 1:1 ratio as the bone graft material. This mixture was combined with whole blood and grafted onto the maxillary sinus (Fig. 2E). The bony window was repositioned (Fig. 2F).
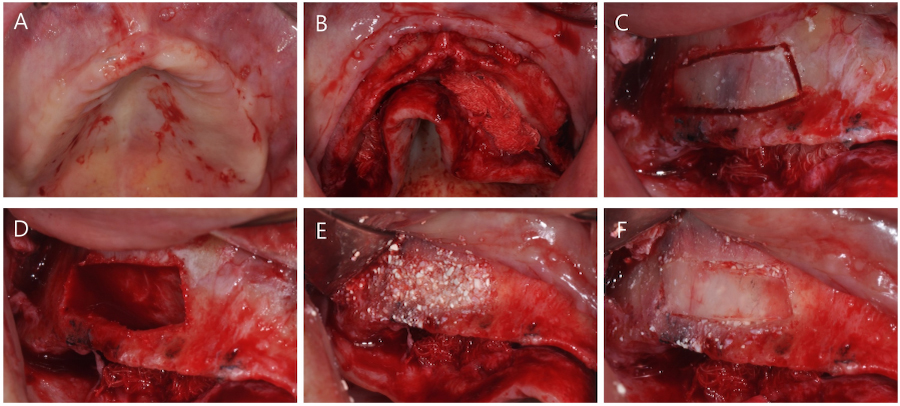
Fig. 2.
Surgical procedure of lateral approach sinus floor elevation. (A) Occlusal view before surgery, (B) Elevation of full-thickness flap, (C) Formation of bony window using a piezoelectric device, (D) Elevation of the Schneiderian membrane, (E) Placement of a mixture of anorganic bovine bone mineral (ABBM) and biomimetic octacalcium phosphate (OCP) graft material, (F) Replacement of the bony window.
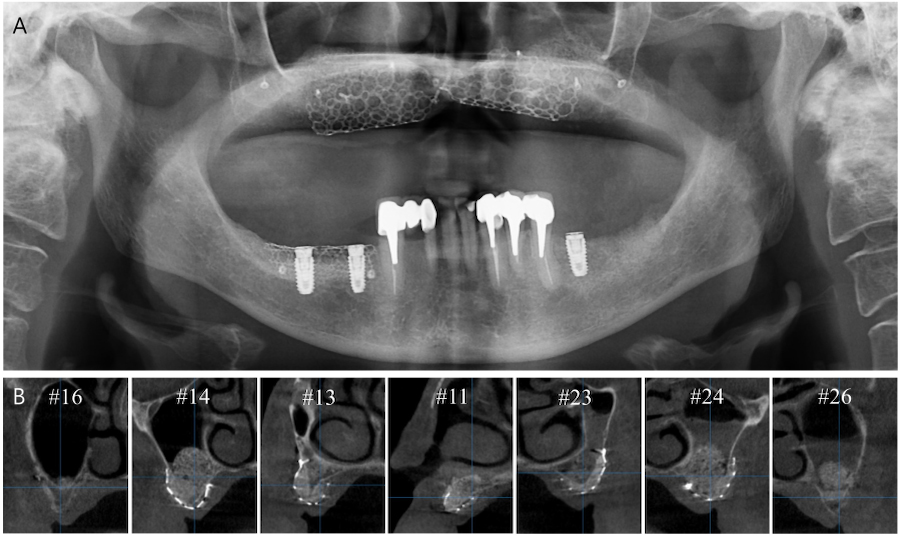
Vertical and horizontal GBR were performed using two titanium (Ti) meshes prepared preoperatively from a three-dimensional printed model (Fig. 3A). The GBR sites were cleaned of all soft tissue remnants and decorticalized using a 330 bur and high-speed handpiece. The Ti meshes were fixed using bone screws (Jeil Medical, Seoul, Korea) (Fig. 3B). The graft material mixtue was placed in the defect (Fig. 3C) and collagen membranes (Ossix Plus; Dentsply Sirona, Charlotte, NC, USA) were placed over the graft (Fig. 3D). Primary closure was then performed (Fig. 3E). Panoramic radiography and CBCT were performed after surgery (Fig. 4). The patient was prescribed antibiotic medication (Augmentin® 625mg; Il-Sung Drug Company, Seoul, Korea) 3 times a day for 7 days.
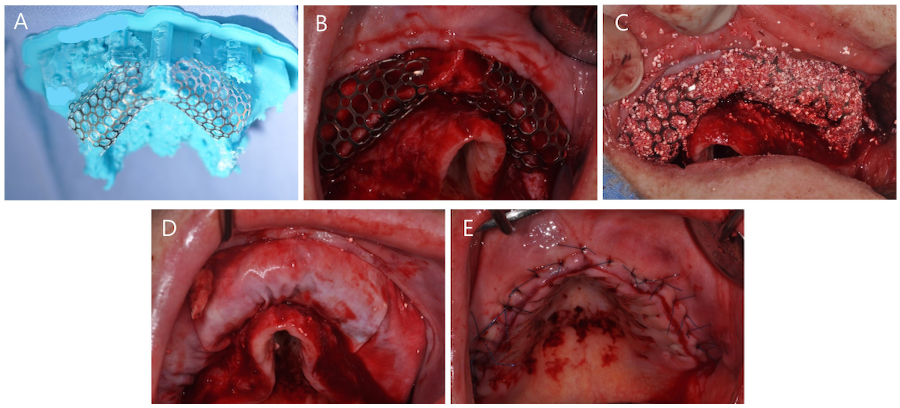
Fig. 3.
Surgical procedure of guided bone regeneration. (A) Two preformed Ti-meshes placed on a three-dimensional cast, (B) The Ti-meshes fixed with bone tacks, (C) Grafting using a mixture of anorganic bovine bone mineral (ABBM) and biomimetic octacalcium phosphate (OCP) graft material, (D) Coverage using collagen membranes, (E) Primary closure. Ti, titanium.
After 5.5 months of uneventful healing, the Ti meshes were removed (Fig. 5A to 5D). Six weeks after Ti mesh removal, digitally guided implant surgery was performed. A core biopsy (diameter 2.0 mm, length 8.0 mm) was harvested using a trephine bur before drilling at site #26 for implant placement, which had previously undergone maxillary sinus augmentation. Subsequently, internal type implants (Osstem TSⅢ; Osstem, Seoul, Korea) were placed using a fabricated surgical guide (Fig. 5E to 5G). Table 1 shows the characteristics of the implants. Vestibuloplasty was then performed via modified periosteal fenestration throughout the maxilla (Fig. 5H). Panoramic radiography and CBCT were performed after surgery (Fig. 6). After 4 months, a provisional prosthesis was delivered (Fig. 7).
Table 1.
Characteristics of implants
| Site | #16 | #14 | #13 | #11 | #23 | #24 | #26 |
|
Diameter (mm) | 5.0 | 5.0 | 4.0 | 3.5 | 4.0 | 5.0 | 5.0 |
|
Length (mm) | 8.5 | 8.5 | 8.5 | 8.5 | 8.5 | 8.5 | 8.5 |
Ⅲ. Discussion
This case report demonstrates the achievement of sufficiently augmented bone dimensions via LSFE and GBR, suitable for implant placement, using a mixture of ABBM and biomimetic OCP. Subsequently, seven implants were successfully placed. During the entire surgical procedure, the patient experienced no notable postoperative complications apart from minor swelling at the surgical site.
LSFE and GBR for vertical and horizontal bone gain have demonstrated predictability in completely edentulous atrophic maxillae.12,13 LSFE has shown favorable outcomes, regardless of the type of graft material used. Various outcomes of GBR have been reported depending on the type of graft material used. In GBR procedures, graft materials serve two primary functions, mechanical and biological.14 They help maintain space, stabilize the blood clot, and support the membrane, while also providing osteogenic, osteoinductive, or osteoconductive effects.
Utilizing a composite graft consisting of autogenous bone chips and a slow-degrading biomaterial, usually a xenograft or allograft, in a 1:1 ratio can offer significant benefits for horizontal and vertical GBR, with several studies demonstrating successful clinical outcomes in terms of regeneration and histological bone formation.15 This approach may also help reduce the high levels of postoperative morbidity, particularly in cases requiring extensive harvesting from the donor site. Additionally, this combination can trigger the release of osteoblasts and growth factors from AB.16 Furthermore, slow-degrading biomaterials undergo gradual resorption, whereas AB facilitates the formation of a newly developed Harversian system, promoting the infiltration of osteoblasts.
OCP, a novel synthetic bone substitute, was developed with the aim of addressing bone regeneration. It has been proposed as a precursor to biological apatite crystals found in bones and teeth, with a crystal structure featuring a water layer between two apatite layers.17 Under physiological conditions, this water layer can be removed, leading to the irreversible conversion of OCP into sustainable biological apatite. OCP has been recognized for its efficacy in promoting new bone formation, which is attributed to its high osteogenic capability and rapid bioabsorption. According to a meta-analysis of sinus augmentation,18 the use of AB alone resulted in a significantly higher rate of newly formed bone (NB) (37.5%), compared with ABBM alone (28.0%, p = .04). However, no significant difference was observed when ABBM was compared with a mixture of AB and ABBM (30.5%, p = .52). In contrast, the current study using a mixture of ABBM and OCP showed a residual OCP graft material (O) rate of 2.9%, indicating substantial absorption of OCP, and an NB rate of 49.5%, demonstrating significant new bone formation (Fig. 8).
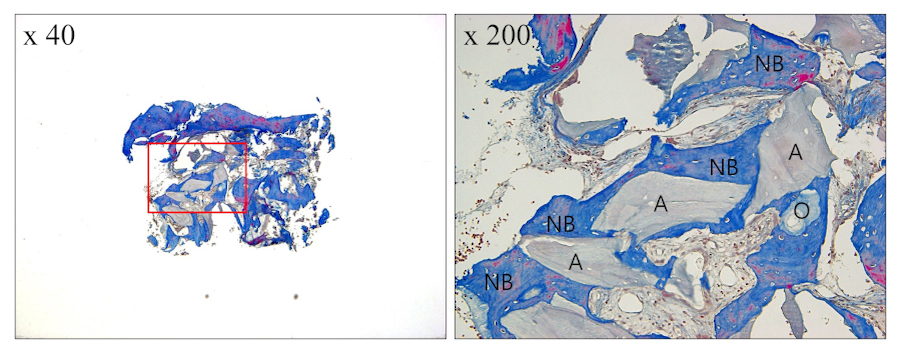
Fig. 8.
Histological examination at site #26 (Masson’s trichrome stained). New bone (NB) is observed with no inflammatory tissue. The histomorphometric analysis results indicated mean proportions of 43.8% for NB, 2.9% for residual octacalcium phosphate graft material (O), 7.1% for residual anorganic bovine bone mineral graft material (A), and 46.2% for connective tissue.
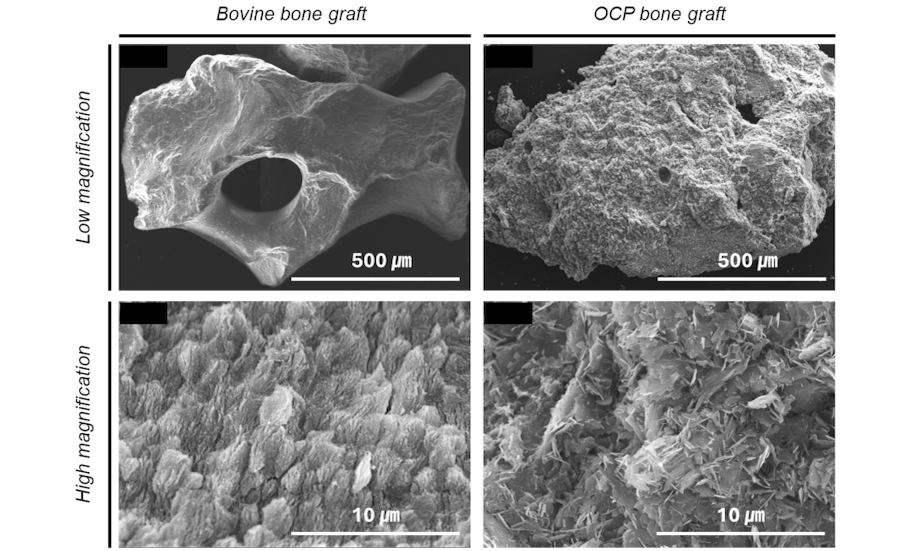
Bontree®, comprising 80 wt.% OCP and 20 wt.% hydroxyapatite (HA), offers unique advantages as it does not undergo the sintering process.19 It can be formed into large solid masses, thereby enhancing its physical properties (Fig. 9). According to Sakai et al., OCP serves as a nucleus for promoting osteogenesis and provides multiple starting points for ossification, distinguishing from HA and β-tricalcium phosphate.20 In our previous study, Bontree® was applied for LSFE in 10 patients, confirming its effectiveness in promoting new bone formation.11 This case report demonstrated favorable results in vertical and horizontal GBR as well as LSFE.