Ⅰ. Introduction
Osseointegration refers to the direct structural and functional connection between the vital bone and the surface of an artificial implant, and is one of the most important factors determining the prognosis of dental implants. Since the discovery by Professor Brånemark of the osseointegration ability of titanium nearly 60 years ago, most dental implants have been made from this material.1 The survival of dental implants is strongly associated with the amount of bone formation directly on the surface of implant. This transmits the resulting forces to the surrounding bones along the implant fixtures, features of successfully osseointegrated implants and their neighboring structures as the functional units that replace the missing teeth.2
To enhance osseointegration, various surface modification techniques have been developed over the past 40 years, with rough-surface implants used today contrasting with the smooth-surface implants that were originally developed.3 The surfaces of implants currently in use are topographically and chemically modified at micro- and nano-levels.3,4 Plethora of evidence supports the clinical success of the titanium root-form endosseous dental implants as the gold standard in oral implantology, and they are commonly used for replacement missing teeth. However, titanium also has certain disadvantages as it can cause discoloration of the peri-implant soft tissue, and hypersensitivity, and may contribute to peri-implantitis. Therefore, alternative materials have been analyzed for their suitability for use in dental implants. One such material is zirconia, which has gained attention owing to its biocompatible, mechanical, and esthetic properties.5
Prior studies in dogs demonstrated osseointegration between stabilized zirconia implants and surrounding vital bones, and favorable correlations with the clinical and histological parameters of the peri-implant tissues.5 The use of zirconia dental implants has been approved in Europe and the United States, but its use in South Korea remains sporadic. In this preliminary in vivo study, we aimed to compare the osseointegration of zirconia dental implants with that of turned-, and rough-surface titanium dental implants.
Ⅱ. Materials and Methods
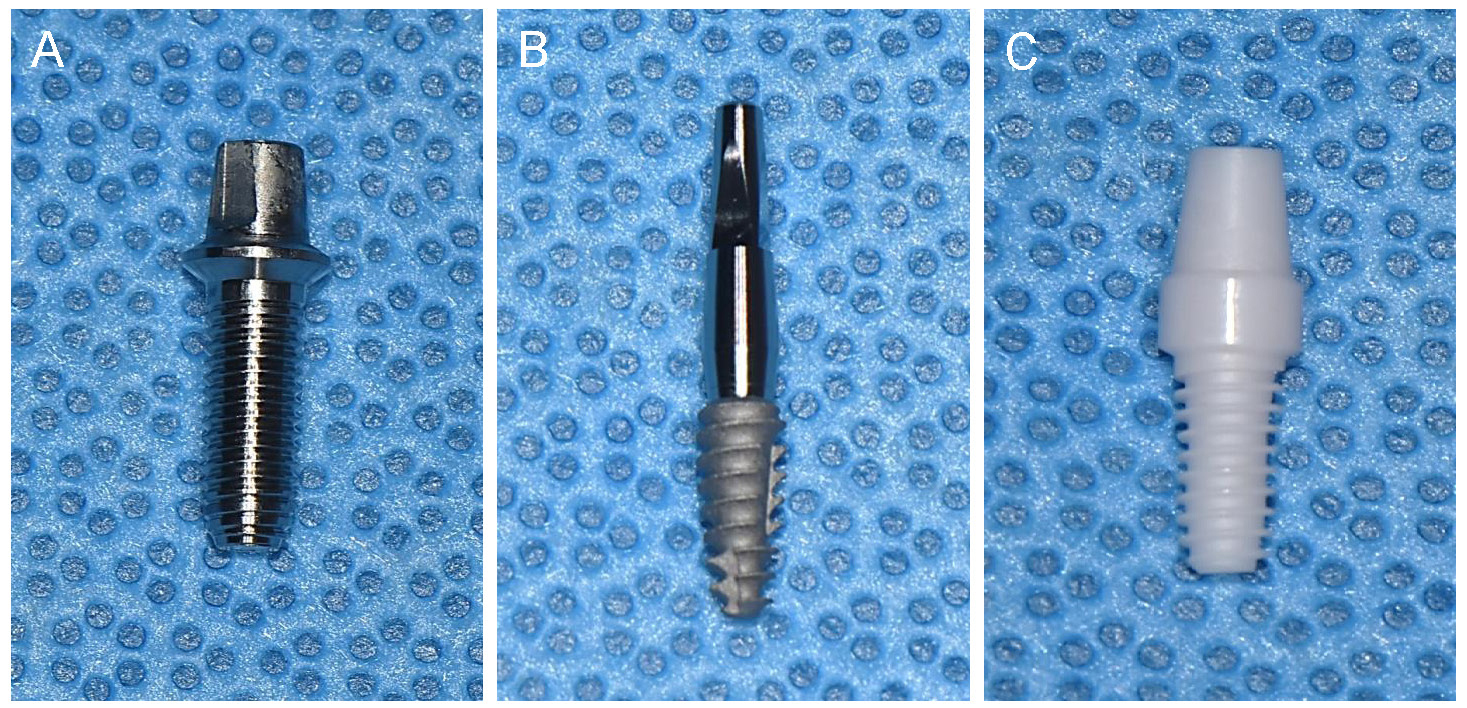
The study was approved by the Institutional Animal Care and Use Committee of Seoul National University (IACUC, SNU-200915-15-2) before the initiation of the current study. Seven healthy 8-month-old beagle dogs, each weighing approximately 12 kg, and with fully developed permanent dentition, were used for in this study. Three types of customized dental implants were used: six turned-surface titanium implants (N = 6, Deep Implant System®, (3.5 mm × 10 mm, Deep Implant System, Seoul, South Korea) (Fig. 1A), six rough-surface titanium implants (N = 6, Neobiotech®, (3.5 mm × 8.5 mm, Neobiotech, Seoul, South Korea) (Fig. 1B), and six zirconia implants (N = 6, Vatech®, (3.4 mm × 8.0 mm, Vatech, Seoul, South Korea)) (Fig. 1C). The rough-surface titanium implants were two-piece systems consisting of implants and abutments, whereas the turned-surface titanium and zirconia implants were one-piece implants.6 The zirconia implants, composed of Zr (83.57%), Y (7.64%), Nb (7.01%), Er (1.02%), and Ce (0.76%), were manufactured using an injection molding process, as described by Yoshinari and Yeo.7,8 This involved blending zirconia powders with stabilizing agents to form a uniform mixture before molding it into the desired shape, and polishing it. The final zirconia implants were cleaned by ultrasonic irrigation and sterilized by autoclaving prior to implantation.
Tooth extraction and implant placement were performed under general anesthesia, which was induced by the intravenous administration of a mixture of tiletamine/zolazepam (Zoletil; Virbac SA, Carros, France) and xylazine hydrochloride (Rompun; Bayer AG, Leverkusen, Germany). Local infiltration anesthesia (Lignospan; Septodont, Saint-Maur-des-Fosses, France) was administered at the extraction and surgical sites immediately before the procedures. Following atraumatic bilateral extractions of the mandibular second, third, and fourth premolars, the extraction sites were allowed to heal for 8 weeks before implant placement. Full-thickness mucoperiosteal flaps were then elevated at the implant sites, and osteotomy sites were prepared according to the manufacturers’ instructions and drilling protocols, using copious saline irrigation. Following implant placement, flaps were primarily closed with resorbable sutures (Fig. 2A to 2C, and 2E to 2G). The implant abutments were adjusted to allow healing without occlusal contact from the opposing teeth.9 The sutures were removed 2 weeks postoperatively. The dogs were monitored at 4-week intervals to assess healing and perform plaque control (Fig. 2D, and 2H to 2K). Signs of inflammation, such as peri-implant mucositis, were managed non-surgically through subgingival curettage and irrigation with saline and chlorhexidine (0.12%) solution where necessary throughout the healing and observation periods.
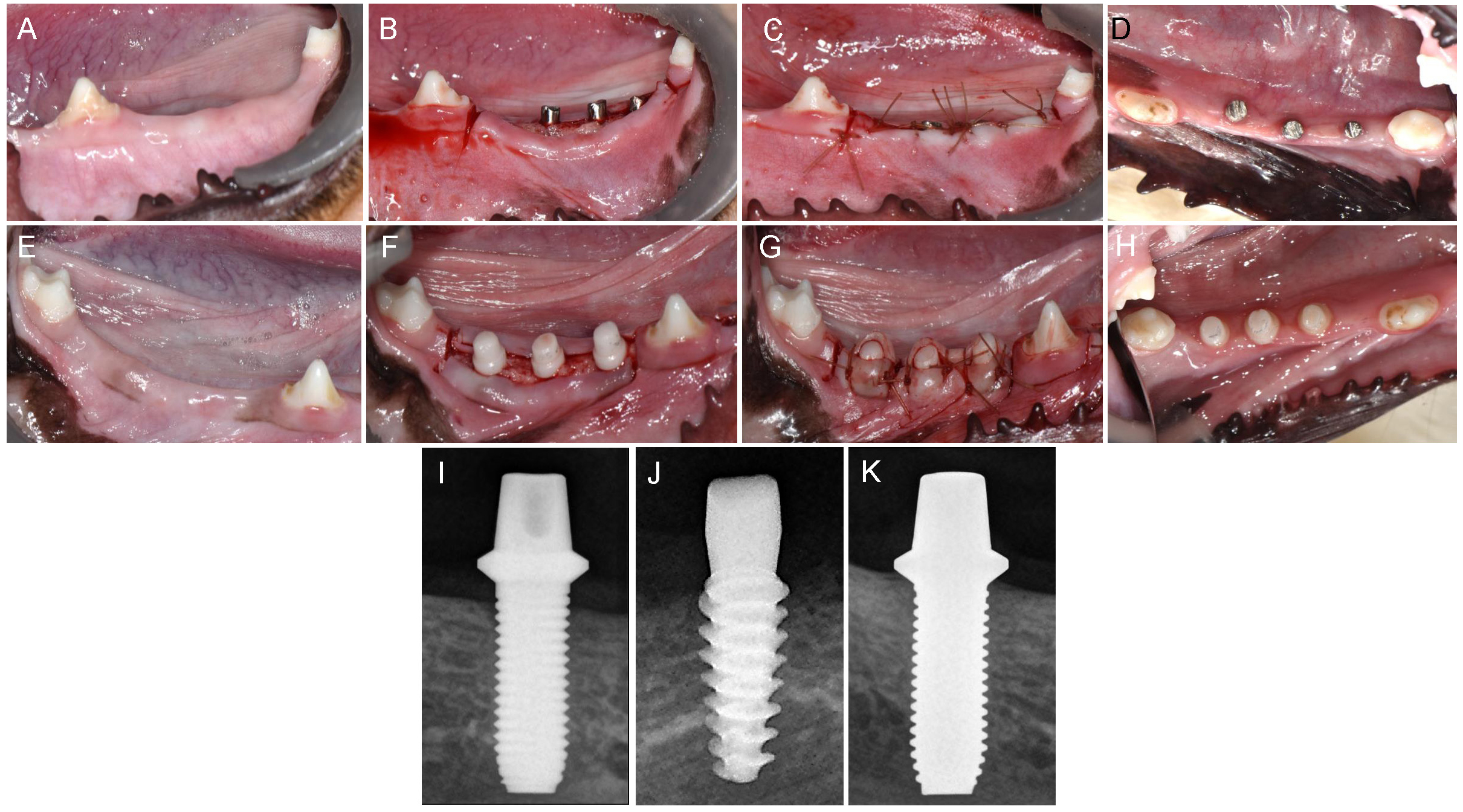
Fig. 2.
Clinical photos and radiographs of implant fixtures. (A) Healed mandibular extraction site, (B) Titanium implants placed and not sutured, (C) Titanium implants placed and sutured, (D) Occlusal view of healed gingiva around titanium implants, (E) Healed mandibular extraction site, (F) Zirconia implants placed and not sutured, (G) Zirconia implants placed and sutured, (H) Occlusal view of healed gingiva around zirconia implants, (I, J, K) Periapical radiographs of turned-surface titanium implant, rough-surface titanium implant, zirconia implant, respectively.
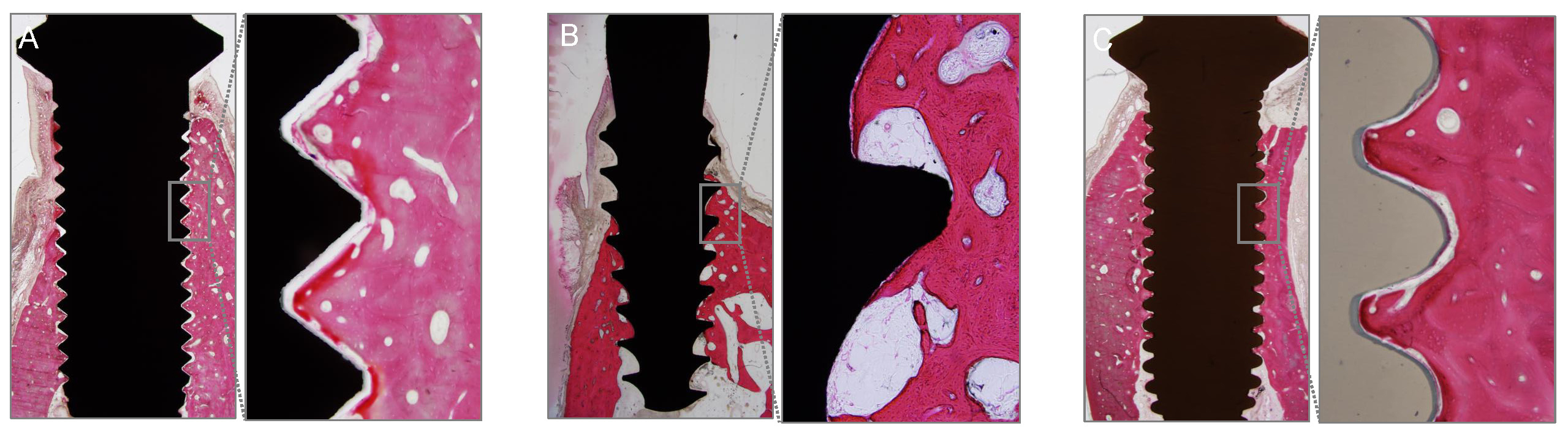
All dogs were euthanized 12 weeks after implant placement, and the implants and surrounding tissues were harvested en bloc to assess osseointegration. Histological slides were prepared for detailed measurements within the designated regions of interest (Fig. 3). Bone-to-implant contact (BIC) and new bone area (BA) were measured under a microscope (Olympus BX; Olympus Corporation®, Tokyo, Japan) using associated software. BIC was determined by measuring the lengths of implant threads in direct contact with the surrounding bone, whereas BA was calculated by measuring the areas of new bone in contact with the implants.
Statistical analyses were performed using SPSS software version 26 (IBM Corporation, Armonk, NY, USA) with the level of significance set p < .05. Data are presented as the mean and standard deviations (SD). The three groups were compared using the ANOVA analysis.
Ⅲ. Results
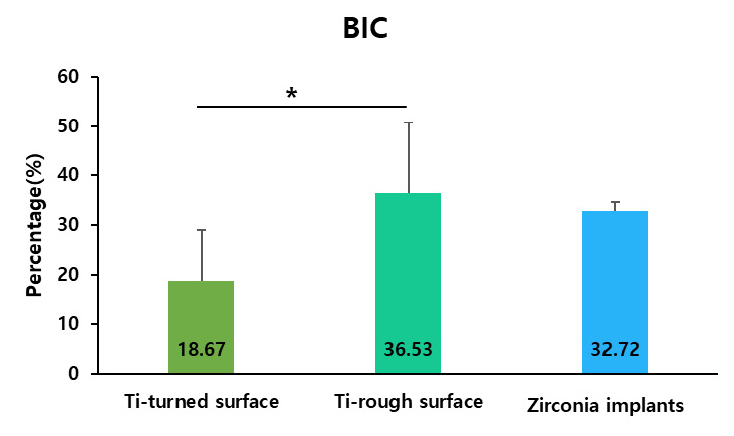
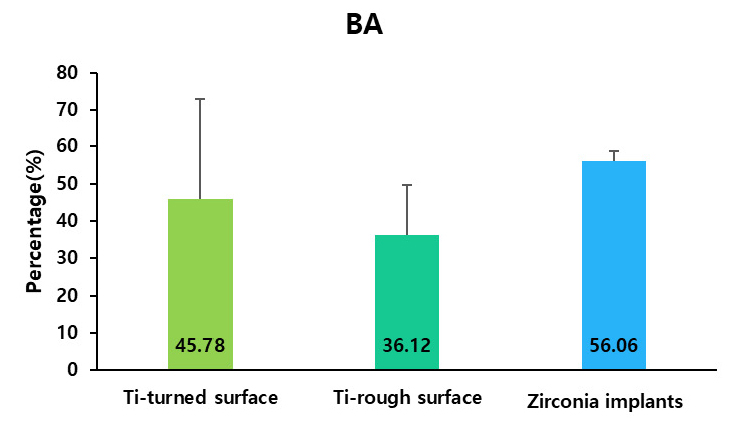
All the implants exhibited uneventful healing, with peri-implant soft tissues healed with no remarks by the Week 2 (Fig. 2A to 2H). Periapical radiographs taken at Week 10 revealed good osseointegration in all groups with no peri-implant pathology or vertical or horizontal bone loss (Fig. 2I to 2K). Table 1 presents the calculated BIC and BA values. BIC differed significantly among the groups, with values of 18.67 ± 10.41%, 36.53 ± 14.23%, and 32.72 ± 2.00% for the turned-surface titanium, rough-surface titanium, and zirconia groups, respectively (p < .05) (Fig. 4). The SDs were relatively similar among the groups. The BA did not differ significantly among the groups, with values of 45.78 ± 27.30%, 36.12 ± 13.41%, and 56.06 ± 2.66% for the turned-surface titanium, rough-surface titanium, and zirconia groups, respectively (Fig. 5). The SDs of the BA values suggested that the use of the turned-surface titanium implants led to more varied results than those from rough-surface titanium or zirconia implants.
Ⅳ. Discussion
This preliminary study showed osseointegration of zirconia implants placed in the mandibles of beagle dogs. The ability to undergo osseointegration is one of the most appreciated features of dental implants, as this process is required for implant stability and function.5 Zirconia dental implants are increasingly considered as alternatives to titanium implants in Europe and North America owing to their esthetic benefits, which are particularly pronounced in individuals with thin gingival biotypes, and the possible risks of peri-implantitis from titanium.10 The present study aimed to assess osseointegration following the placement of zirconia or titanium implants to provide evidence to support the future development and clinical application of zirconia implants in South Korea.11 The results showed comparable or enhanced levels of osseointegration, as determined by BIC and BA within the regions of interest, following placement of zirconia implants compared to turned- or rough-surface titanium implants. Regions of interest were selected from anywhere within the coronal halves of the fixture samples to account for potentially suboptimal osseointegration. The turned-surface titanium implants showed relatively lower levels of osseointegration than the other two groups, with the rough-surface titanium and zirconia groups showing comparable osseointegration levels. This indicates that the zirconia implants are equal to the currently used rough-surface titanium implants in terms of osseointegration in the mandibular ridges of beagle dogs.12
One-body turned-surface titanium implants were used in this study to facilitate comparison with readily available one-piece zirconia implant.13 However, a two-piece rough-surface titanium implant was chosen as this is the most commonly used implant type in current clinical practice. We, therefore, believe that these results provide an approximate level of osseointegration for the former and current most frequently used dental implants.
BIC and BA results following zirconia implant placement are in agreement with those reported by previous studies.14 Hoffmann et al. reported BIC and BA values of 41.35% and 38.40%, respectively, following the placement of zirconia implants into the femur bones of rabbits.5 Surface modification of zirconia implants altered the BIC and BA with laser and sandblasting leading to higher bone apposition.8 Yeo’s recent study compared surface-modified zirconia and titanium implants, with similar findings.8,15 Significantly higher BIC values were observed with rough-surface titanium implants, modified by the sandblasted and acid-etched (SLA) technique, than with smooth-surface titanium implant.16 These findings are similar to the results of our study, in which BIC values were similar in zirconia and rough-surface titanium implants and lower in turned-surface titanium implants, whereas BA levels were similar among all groups. However, the relatively small number of animals used in the present study means that the results should be extrapolated with caution.
The findings of our study therefore appear consistent with those of prior reports on the osseointegration of titanium and zirconia implants.17 However, this study has certain limitations. The number of animals used was small, and histological characteristics of osteoblastic and osteoclastic activities were not investigated. These will be examined in future studies. Further surface modification and enhancement of zirconia implants, supported by evidence of their long-term survival should enhance their clinical use.
Ⅴ. Conclusion
Within the limitations of this study, the results suggest that zirconia implants become biologically integrated into the healed edentulous mandibular ridges of beagle dogs. This integration should allow functional loading, although further studies are required to demonstrate this. Moreover, future investigations on the microbial adherence and susceptibility to peri-implantitis of zirconia implants are needed to increase their clinical application.