Ⅰ. Introduction
Ameloblastoma is often characterized by bone invasion and the presence of multiple lesions, which significantly increases the risk of recurrence when treated conservatively.1 Large lesions can result in pronounced jaw deformities, leading to occlusal disorders and pathological fractures that significantly impair normal mastication.1 Meticulous treatment planning is essential in cases of extensive lesions and important anatomical structures. For lesions causing severe mandibular deformities, a two-stage approach involving initial decompression followed by surgical intervention is recommended.2
Considering the complexities associated with significant mandibular defects, reconstruction using a vascularized fibula free flap (FFF) has gained popularity owing to its robust vascular supply.3 Additionally, osseointegration between the fibula and implants has demonstrated a prognosis comparable to that of the native mandible.4 However, a common concern following FFF reconstruction is the height discrepancy between the fibula and native mandible.5This height difference can complicate dental implant placement and negatively affect the overall aesthetic and functional outcomes.
Herein, we present an approach that optimizes the crown-to-implant ratio by positioning the fibular graft at the mid-height of the mandible. Additionally, we demonstrated that preserving the temporomandibular joint (TMJ) disc during mandibular condyle resection prevents joint ankylosis. The implant-related outcomes of this reconstruction approach are emphasized, highlighting the achievement of stable occlusion and detailing the bony changes observed in the neo-condyle at the long-term follow-up.
Ⅱ. Case Report
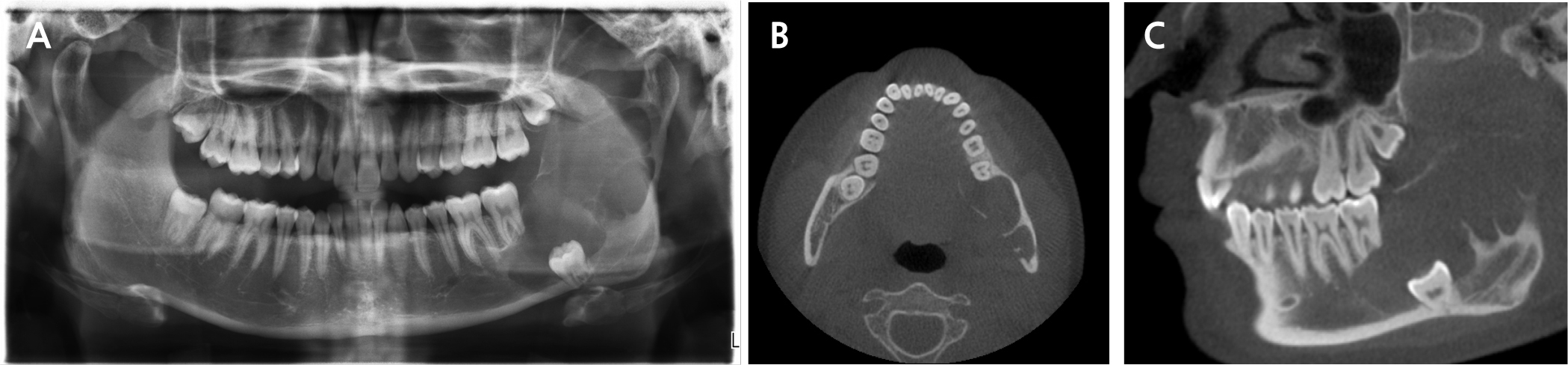
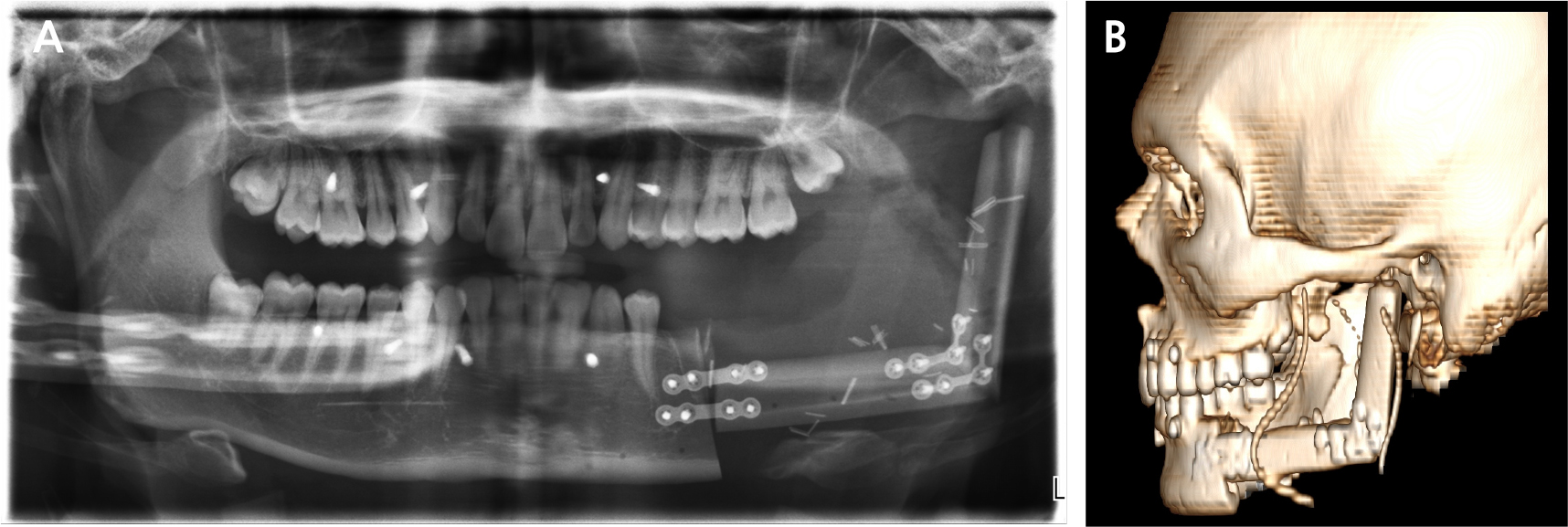
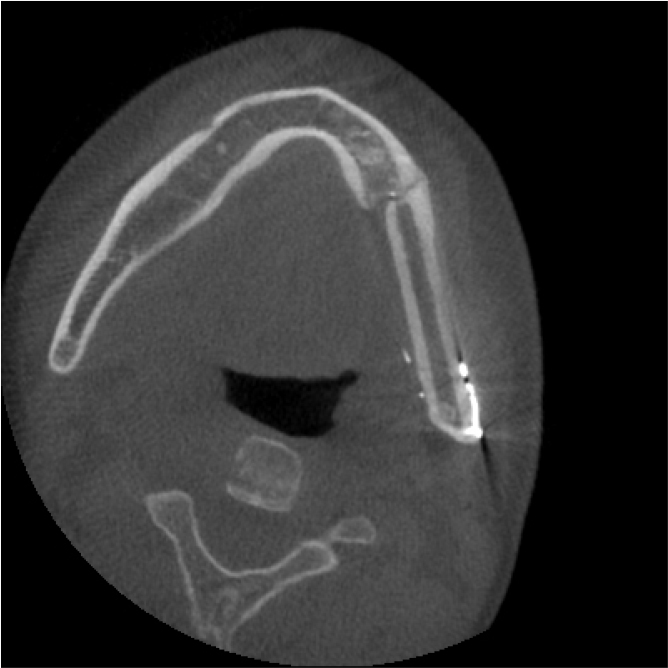
A 21-year-old male presented to the Department of Oral and Maxillofacial Surgery at Gil Medical Center on February 21, 2019, with pain in the left mandible and difficulty in opening his mouth. Clinical examination revealed hypoesthesia in the left lower lip and a maximum mouth opening of 10 mm. Panoramic radiography revealed a multilocular radiolucent lesion extending from the posterior region of the left mandibular second molar to the left ramus, causing displacement of the left third molar and root resorption of the second molar (Fig. 1A). Cone beam computed tomography (CBCT) images confirmed cortical bone expansion in the left mandible and displacement of the left inferior alveolar nerve canal (Fig. 1B and 1C).
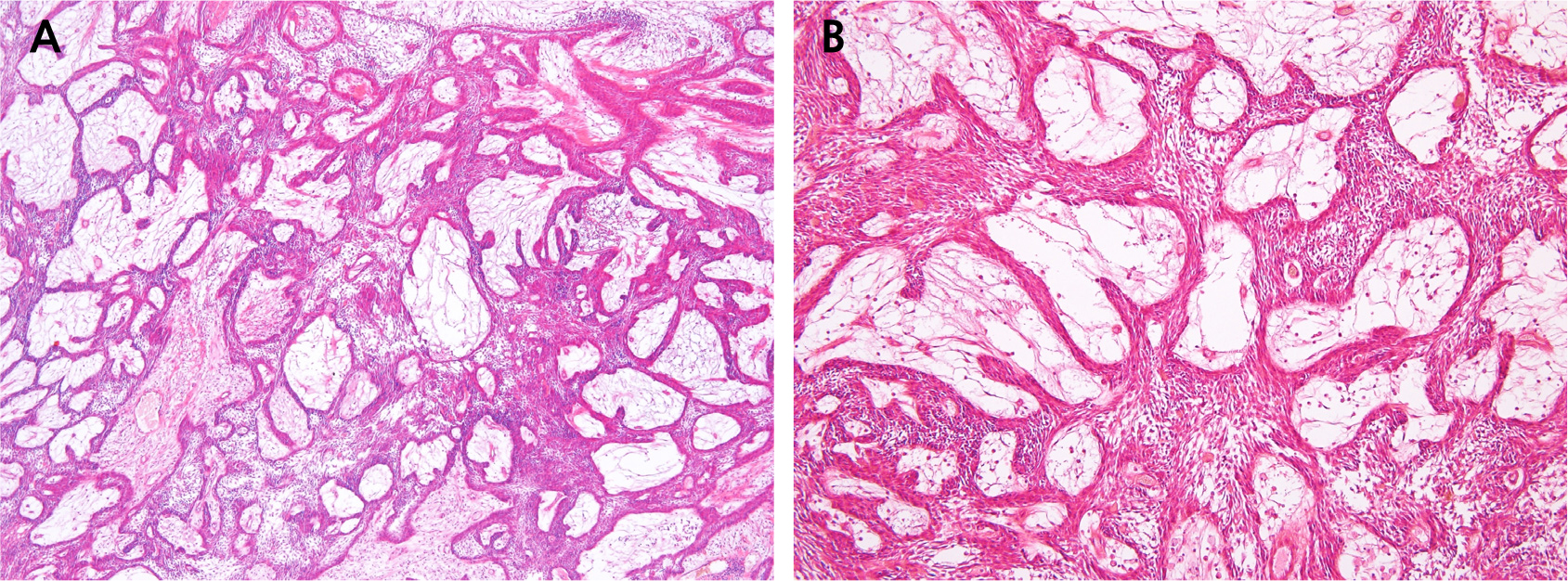
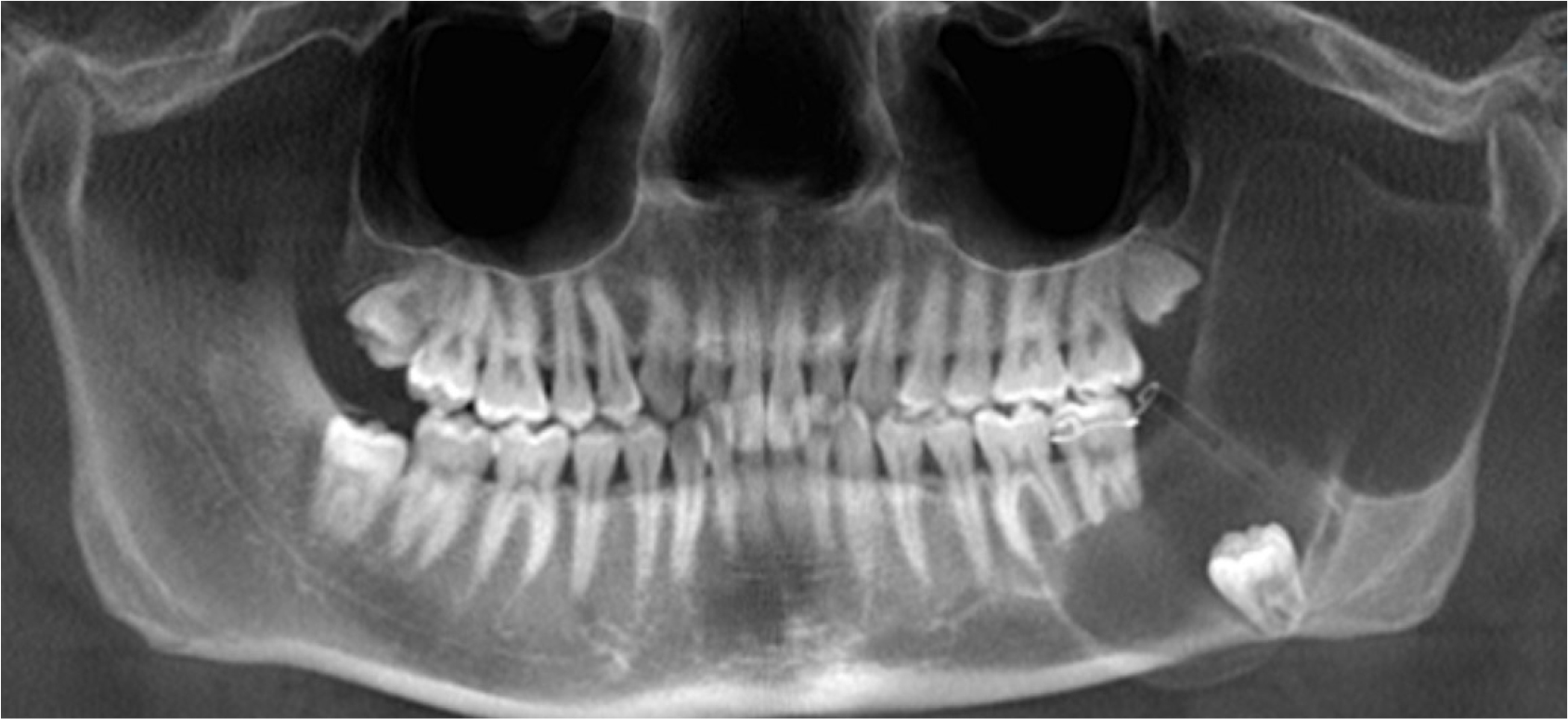
On the same day, decompression and incisional biopsy were performed under local anesthesia. Two drains were placed distal to the left second molar for decompression. The biopsy specimen measured 1 × 0.5 cm, and the intralesional area was filled with clear exudate. Histopathological examination revealed a plexiform-type ameloblastoma characterized by multiple epithelial islands intricately connected in a complex, web-like structure. (Fig. 2A and 2B). Decompression was successfully maintained, leading to a significant reduction in lesion size. However, 6 months later, a follow-up radiograph image indicated slight lesion enlargement (Fig. 3).
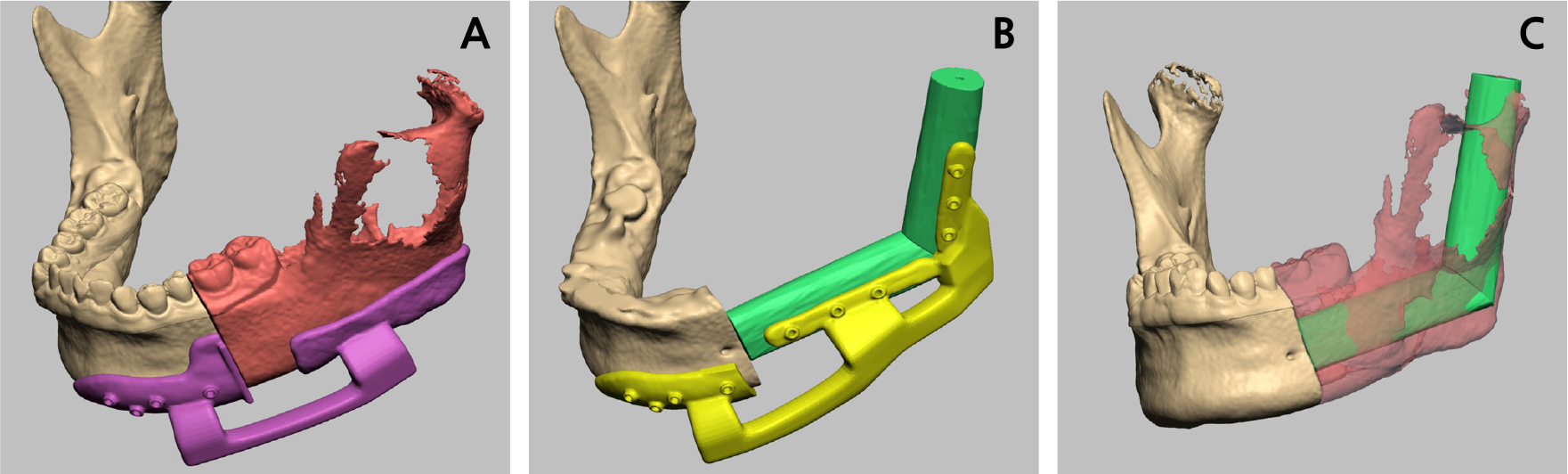
A left partial mandibulectomy was performed under general anesthesia, followed by reconstruction using a vascularized FFF. Preoperatively, a head and neck computed tomography (CT) scan and angiography of the lower leg were performed to assess the blood circulation in the lower limb. Prior to surgery, virtual surgical planning (VSP) was performed using Mimics software (version 19.0; Materialise NV, Leuven, Belgium). During VSP, we planned to resect the left mandible from the posterior part of the second premolar, extending to the ramus, including the condyle. A cutting and positioning guide was created based on the VSP (Fig. 4A and 4B). A virtual left partial mandibulectomy was performed, and the fibula model was positioned to reconstruct the defect, aligning with the natural shape of the mandible (Fig. 4C). The fibula bone graft was connected to the mid-height of the recipient mandible.
On September 5, 2019, the following surgical procedures were performed under general anesthesia:
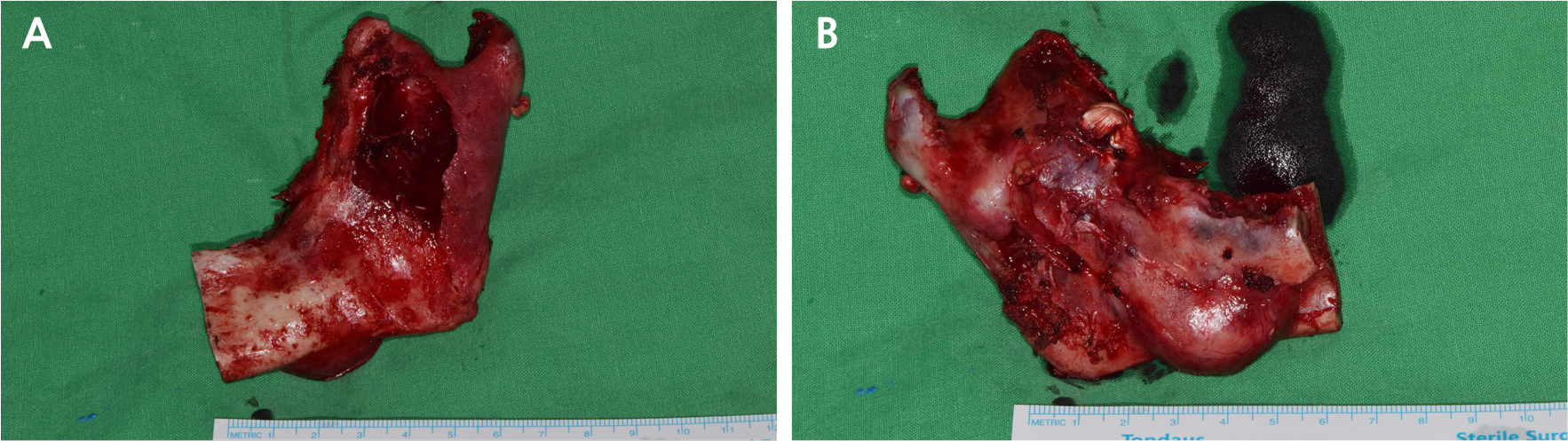
• A submental flap extending to the left submandibular region was created for optimal access. Following the preoperative plan, left partial mandibulectomy was performed using a cutting guide, extending from the posterior region of the second premolar to the mandibular condyle. The left TMJ disc was preserved, and the left inferior alveolar nerve was ligated. Cortical expansion and partial perforations were observed on the buccal and lingual sides of the resected mandible (Fig. 5A and 5B).
• A tourniquet was applied to the thigh to restrict blood flow, the flap area on the left leg was marked, and an osseous flap, rather than a skin flap, was created. Using a prefabricated guide, the fibula was precisely cut above the ankle and below the patella and subsequently shaped according to the preoperative plan for mandible reconstruction. Microvascular anastomosis was performed from the peroneal artery to the left facial artery and from the vena comitans to the facial vein.
• The prepared fibula graft was positioned using the guide and secured with miniplates at the mid-height of the recipient mandible (Fig. 6A and 6B). The distal fibular segment was positioned within the glenoid fossa (Fig. 6B). The surgical site was closed using layer-by-layer suturing and negative pressure drains were placed to prevent hematoma formation.
Malocclusion was observed postoperatively, prompting the application of intermaxillary fixation (IMF) with rubber rings for the first two weeks, followed by nighttime use for an additional two weeks. After 1 month of using guide elastics, the occlusion was nearly restored to its preoperative state, and the pain was significantly reduced.
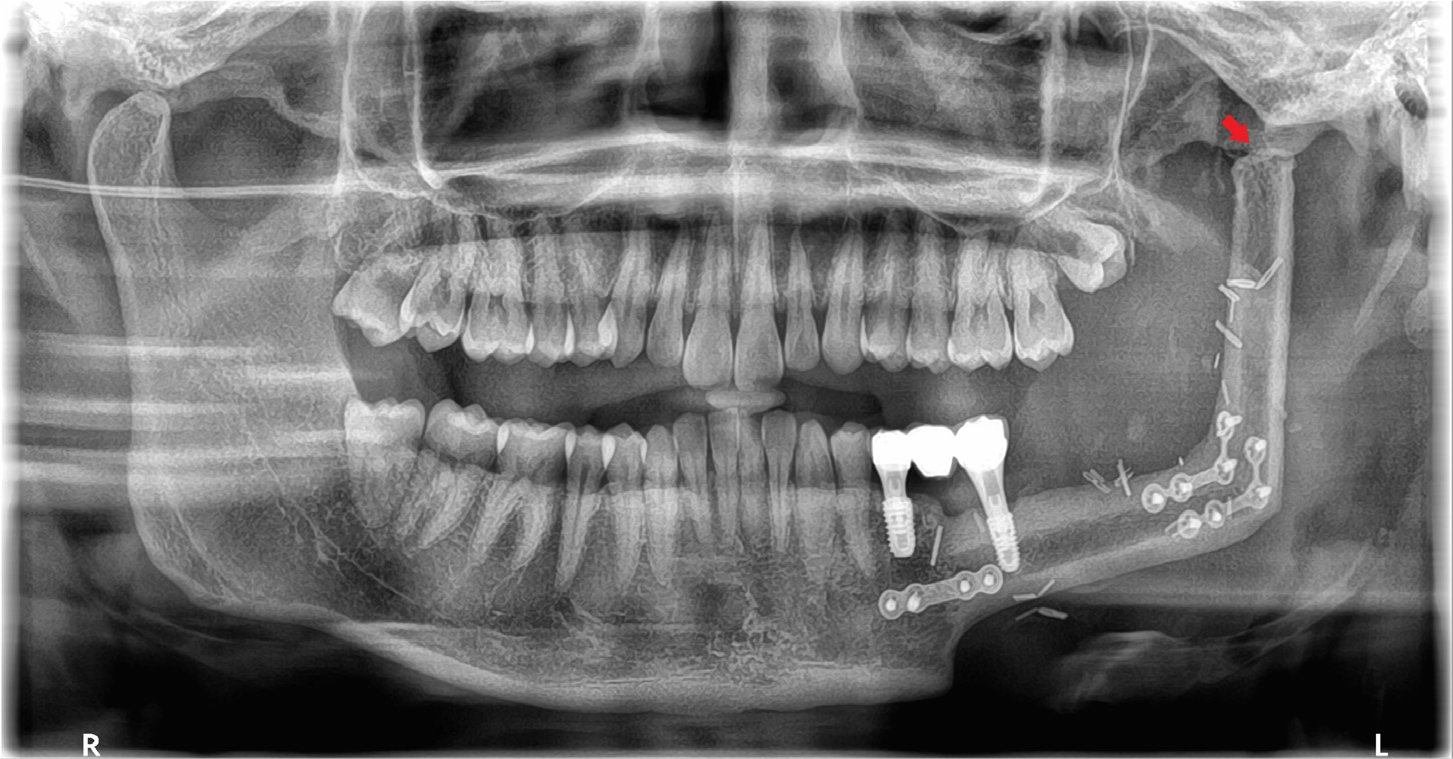
At the 8-month postoperative follow-up, signs of bone union were observed at the graft site without evidence of recurrence (Fig. 7). Hence, implant rehabilitation was performed in the mandible reconstruction area. Implant positions were planned, and a surgical guide was created using CBCT and a three-dimensional model of the mandible.
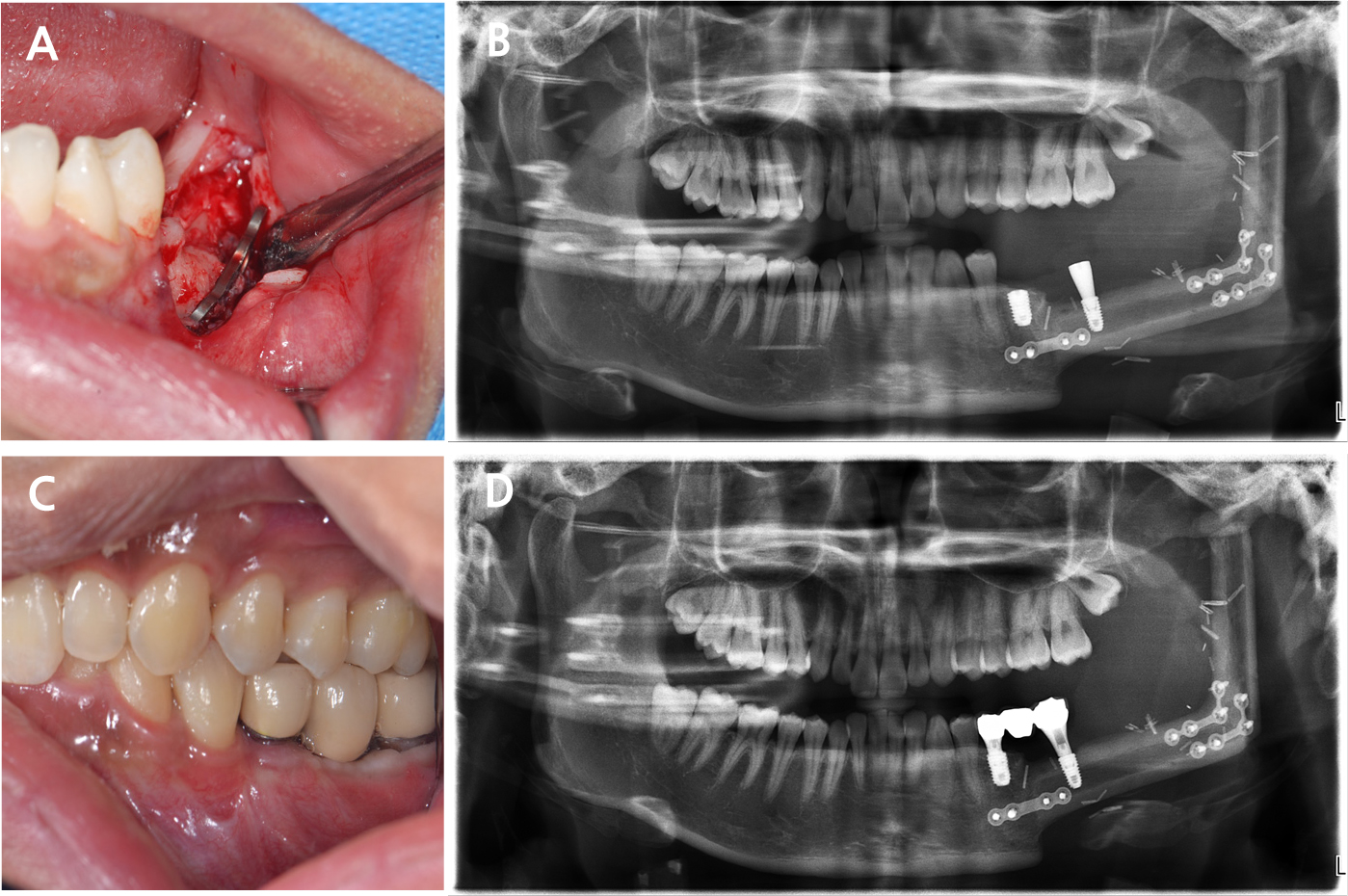
Two implants (left second premolar: TS III 5.0 × 8.5 mm and left second molar: TS III 4.5 × 8.5 mm; Osstem, Seoul, Korea) were placed using the created guide under local anesthesia. Before implant placement, the overlying metal plate obstructing the implant site was removed. Visual inspection confirmed complete bone union between the fibula and mandible (Fig. 8A). Notably, the implant for the second premolar was placed in the native mandible, whereas that for the second molar was placed in the fibula bone. Despite sufficient bone volume, the second premolar implant was submerged owing to poor initial stability. Conversely, the second molar implant exhibited good initial stability, allowing for the placement of a healing abutment (Fig. 8B). Second-stage surgery of the second premolar was performed 3 months after the initial implant placement. Two months after the second-stage surgery, non-hex customized abutments were tightened to 30N, and a porcelain-fused-to-metal crown and bridge were placed. The final prosthesis was designed to aesthetically match the adjacent teeth while ensuring that the crown-to-root ratio of the implant placed in the fibular bone remained within acceptable limits (Fig. 8C and 8D).
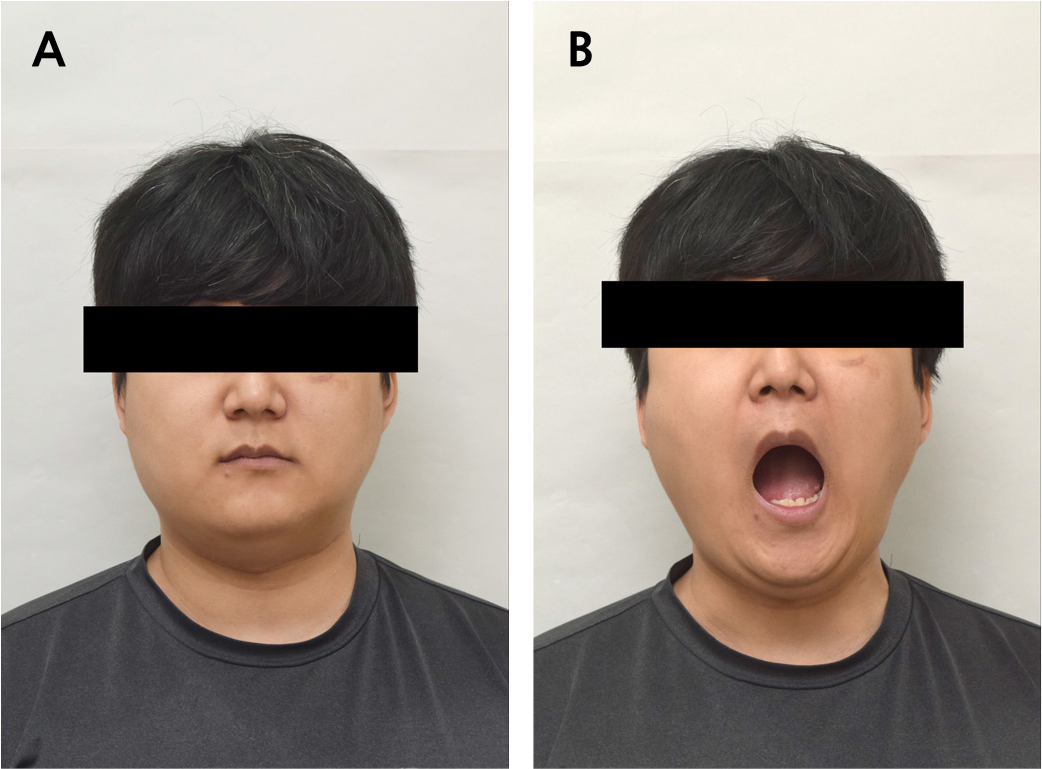
During the 55-month follow-up period, no recurrence or complications occurred at the site of mandibular reconstruction. The implant site showed stable osseointegration, with no prosthetic issues, such as prosthetic fracture or screw loosening. After implantation, the FFF-reconstructed neo-condyle became rounded, resembling the natural condyle as the cortical lining formed (Fig. 9). The mandibular contour showed improved symmetry (Fig. 10A), and the patient showed stable occlusion with significant clinical improvements in mouth opening and masticatory function (Fig. 10B).
Ⅲ. Discussion
Ameloblastoma often involves bone invasion and multiple lesions, increasing the likelihood of recurrence when treated conservatively.1 A two-stage approach consisting of decompression followed by surgical enucleation with curettage is recommended for patients with extensive ameloblastoma, resulting in severe jaw deformity.2 Decompression reduces the pressure exerted by the lesion on the bone tissue, promoting a balance between osteoclast activity and bone remodeling.1,6Once a new equilibrium is reached, the lesion size stops decreasing, making it suitable for definitive surgical intervention.1,2 In this case, the lesion size was initially reduced by decompression, and subsequent enucleation or resection was planned. Although decompression initially reduced the lesion size, an increase was observed during follow-up. Hence, surgical resection was performed to minimize the risk of recurrence.
In the present case, the lesion was extensive and involved critical anatomical structures, requiring precise treatment planning to ensure both functional and aesthetic outcomes. VSP played a crucial role in preoperatively mapping the resection and reconstruction sites, as well as determining the optimal length and width of the FFF. The use of three-dimensional reconstruction for preoperative evaluation has recently become the gold standard for designing accurate osteotomies and selecting the most suitable flap and fixation methods.7 VSP significantly enhances the accuracy of reconstruction, particularly in terms of anatomical alignment, thereby contributing to the success of the procedure.8 Custom cutting and positioning guides were developed during the VSP phase. Surgery was performed as planned using these guides to ensure precise reconstruction. The procedure involved mandibular resection, including the left mandibular condyle, followed by reconstruction with a vascularized osseous FFF.
After reconstruction, the prognosis of the graft was assessed, and dental rehabilitation with implants was considered. The fibula is a well-established donor site for mandibular reconstruction owing to its robust vascular supply and predictable osseointegration of implants.3 However, the optimal period between reconstruction and implant placement remains controversial.9Bodard et al. advised against immediate implant placement during reconstruction due to insufficient surgical guidance and clinical criteria for ensuring accurate implant positioning.10De Santis et al. proposed a two-stage approach, where mandibular reconstruction is initially completed, followed by implant placement after a healing period of approximately 6 months.11 This staged protocol facilitates optimal bone remodeling and soft tissue maturation, reduces the risk of implant failure, and improves long-term functional outcomes.11 In our case, a 9-month period following reconstruction was selected before proceeding with guided implant placement. This approach allows precise implant positioning, both functionally and anatomically, ensuring better stability and long-term success. The guided implant system provided the necessary accuracy for optimal prosthetic rehabilitation, further supporting the benefits of delayed implantation in patients with complex reconstruction. Additionally, by positioning the fibula graft at the mid-height of the mandible, we optimized the crown-to-implant ratio. In FFF reconstruction, the most common concern is the height discrepancy between the fibula and native mandible.5 Typically, the fibula is aligned with the inferior border of the mandible to restore its profile.12 However, this traditional approach often requires excessively long prostheses to reach the occlusal plane. The resulting unfavorable lever arm forces place excessive stress on the implants, thereby increasing the risk of implant complications.13 Conversely, our approach not only reduced the length of the prosthesis but also minimized the functional complications associated with excessive implant forces, leading to improved long-term outcomes.
A defect extending to the left mandibular condyle was anticipated when planning partial mandibulectomy. Hence, the TMJ disc was preserved during resection of the left mandibular condyle. Previous studies have reported that preserving the TMJ disc allows the remaining disc to function as a biological spacer, preventing stiffness and facilitating functional remodeling of the reconstructed neo-condyle within the glenoid fossa.14,15 Park et al. reported that when the disc was preserved, the distal end of the fibula became rounded, and the neo-condyle gradually realigned within the glenoid fossa by the 3-month postoperative follow-up.16 In the current case, the distal end of the fibula gradually reshaped, resembling the original morphology of the mandibular condyle during follow-up, further supporting the benefits of TMJ disc preservation.
Following FFF reconstruction, condylar bone growth can occur toward the lateral pterygoid traction direction and glenoid fossa. In both directions, the TMJ disc separates the neo-condyle bone growth from the glenoid fossa, preventing postoperative dysphagia or ankylosis.17 To further minimize the risk of ankylosis, the fibula should be positioned within the joint space without direct contact, and early application of guided elastics should be initiated after surgery.18 Additionally, early postoperative mobilization of the TMJ preserves its function and facilitates rehabilitation.19 In this case, IMF with elastic rubber rings was applied for two weeks, followed by nighttime use of elastics for an additional two weeks to allow joint movement. This approach is likely to have contributed to the improvement in the mouth opening. In conclusion, TMJ disc preservation is critical in preventing new bone growth resulting in TMJ ankylosis after FFF reconstruction.15,17
Ⅳ. Conclusion
In this case, mandibular reconstruction with FFF using VSP resulted in long-term implant stability and optimal functional outcome. VSP was employed to align the fibula flap vertically on the native mandible, ensuring accurate positioning during surgery. Considering that future implant placement is a key consideration, the fibula flap was strategically positioned to facilitate this. TMJ disc preservation is essential for preventing ankylosis and supporting neo-condyle remodeling. These findings highlight the significance of precise surgical planning and anatomical preservation in achieving optimal mandibular rehabilitation.