Ⅰ. Introduction
Dental implant therapy is a well-established method to replace missing teeth.1 However, various types of failure and adverse effects are observed with popular use of implants. Peri-implant diseases including peri-implant mucositis and peri-implantitis have become one of the most frequent complications affecting the tissues around implants which can lead to the loss of the implants.2, 3 It is generally accepted that the microbial biofilm plays an analogous role in the development of peri-implant inflammation as colonization
follows a similar principle at teeth and implant surface.3, 4 Despite limited data, peri-implant mucositis and peri-implantitis have been reported to affect 50% and 12-43% of the implants, respectively. 5 Clinically, peri-implantitis sites exhibit signs of inflammation, bleeding on probing (BOP), and/or suppuration, increased probing pocket depths (PPD), and/or recession of the mucosal margin. Also, bone loss is shown radiographically compared to previous examinations, unlike peri-implant mucositis, which affects only soft tissue around implant.3, 4
Various non-surgical and surgical treatment have been introduced for the control of peri-implant disease. Non-surgical treatment for peri-implant mucositis is effective for reducing inflammation of peri-implant tissue.3, 45 However, for peri-implantitis, non-surgical treatment showed limited improvements in the main clinical parameters and there was a tendency to recurrence of the disease because of insufficient implant surface disinfection.5 Generally, surgical treatment includes mechanical debridement,2, 6 chemical decontamination,2, 7 local administration of antibiotics,번호8 and regenerative procedure using bone graft.5, 9 These can be applied simultaneously under open flaps and/or done in multiple combinations. In a retrospective study by Langervall et al.,10 it was shown that 84% of the open flap debridement had been successful at implant level.
Surgical treatment with regenerative procedure using bone graft shows statistically significant improvements in radiographic bone fill compared to open flap debridement alone.11 Although a partial defect fill can be expected, but complete fill of intrabony defects caused by peri-implantitis using a guided bone regeneration (GBR) seems not to be a predictable outcome.12
Most studies of peri-implantitis treatment reported only short-term results. Also reference for domestic condition is hard to find. Therefore, the purpose of this case report was to radiographically evaluate the long-term effect of surgical treatment of peri-implantitis defects using regenerative bone graft procedure for more than 3 years after operation.
Ⅱ. Case
1. Case 1
A 71-year-old male patient visited the department of periodontology because of implant loss and peri-implantitis on left posterior mandible. He had received implant surgery on #35, #36 3 years ago, but had been suffering from swelling and pus around implants for 2 years, and finally lost #36 implant spontaneously the day before visiting the hospital.
Probing pocket depth (PPD) and radiographic bone defect depth was measured at baseline and over 3
years after surgery. Marginal bone defect was measured via distance of implant fixture-abutment interface (IP) to most coronal point of bone to implant contact (BIC) (IP-BIC).
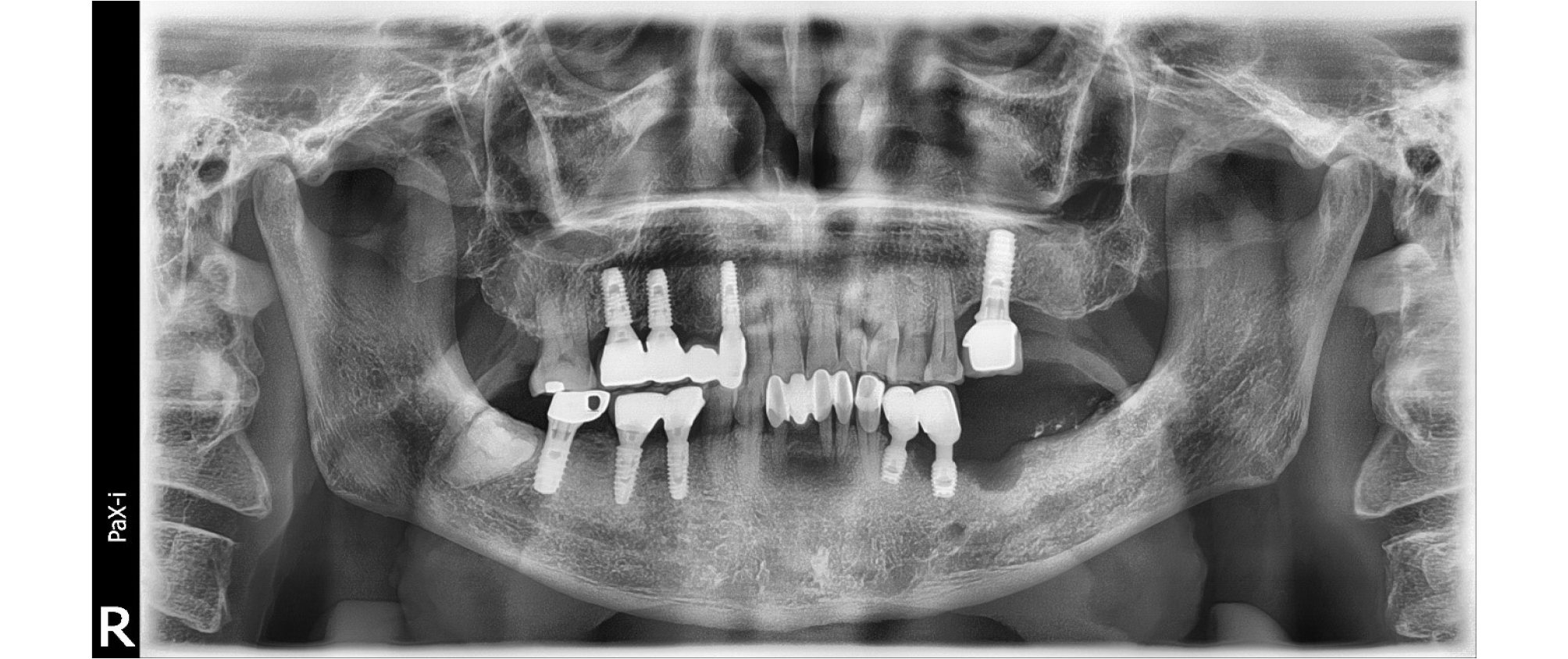
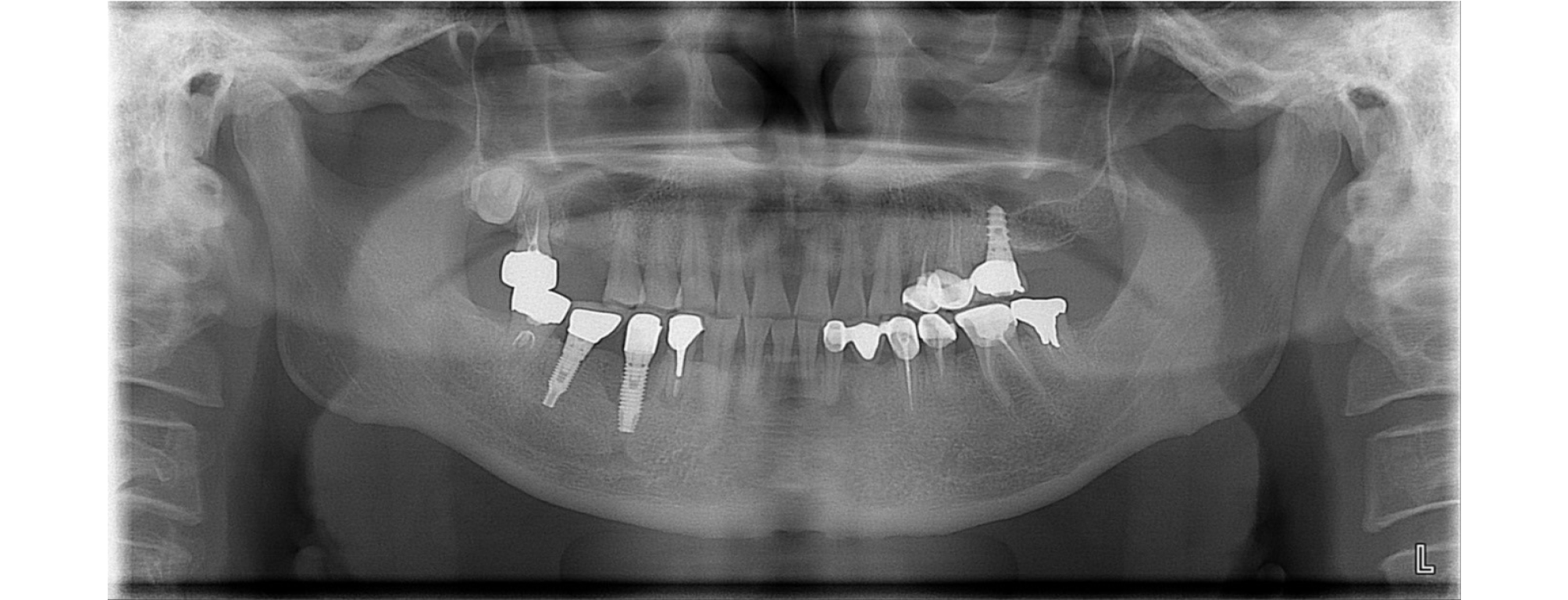
A 6 mm PPD with 1 mm peri-implant mucosal recession was observed at the lingual side of #35 implant (#i35). Additionally, BOP and pus discharge were observed. Implant mobility was not observed. Marginal bone defect of 6mm depth from IP was observed at mesial site of #35 implant, and large radiolucent lesion due to loss of #36 implant was detected on the baseline panoramic radiograph (Fig. 1).
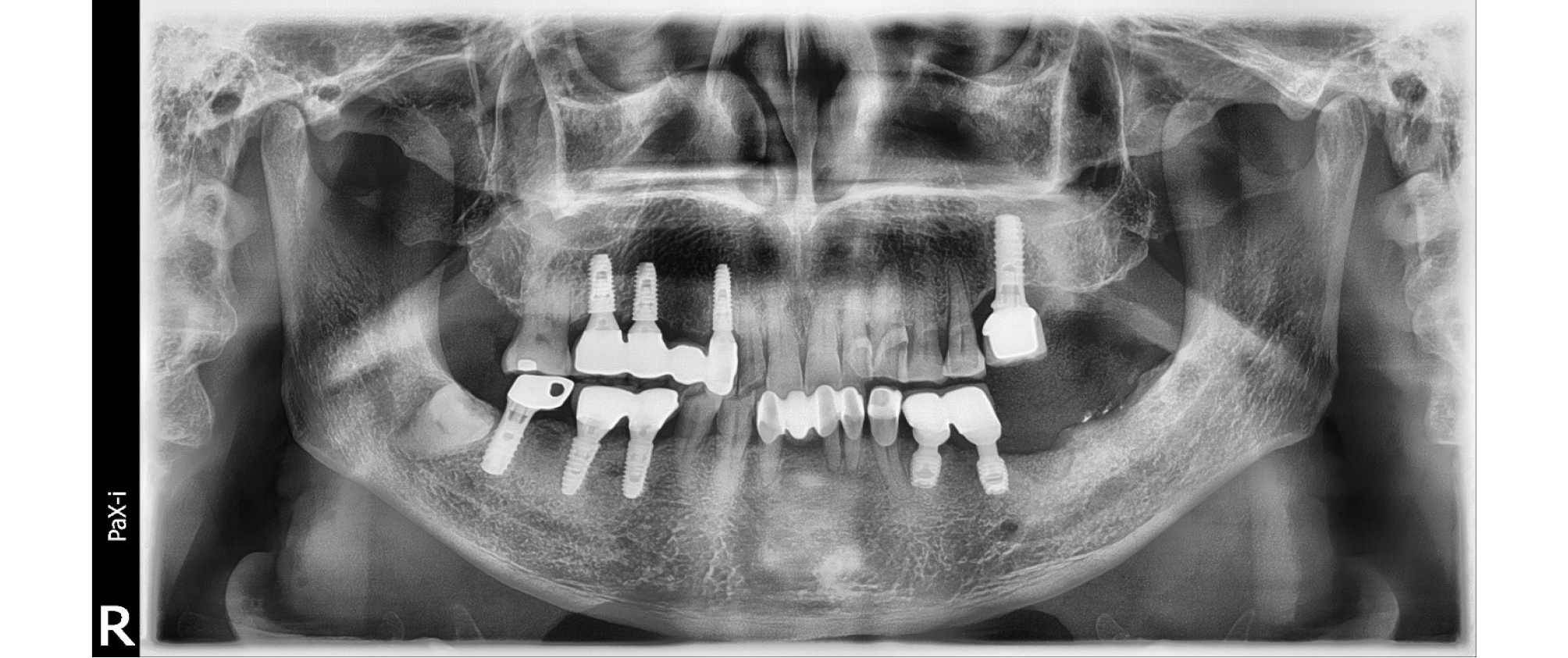
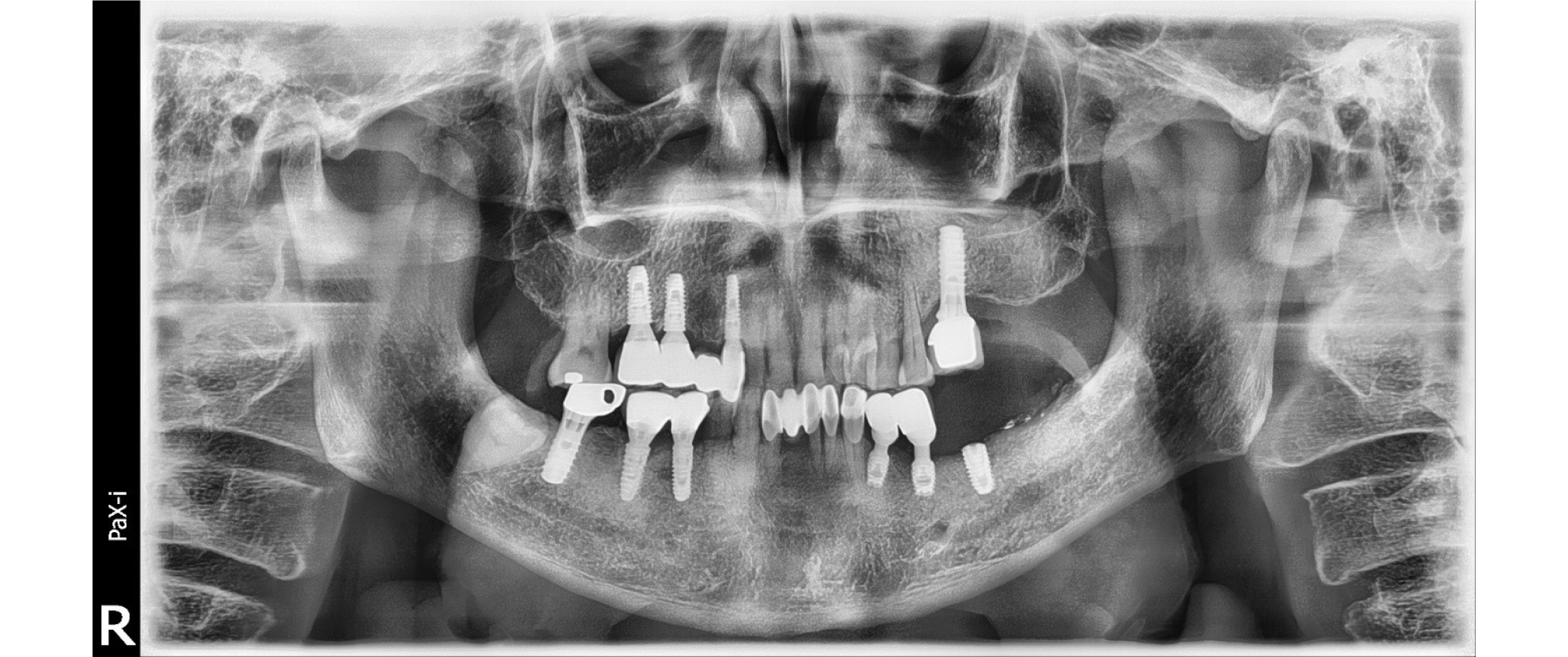
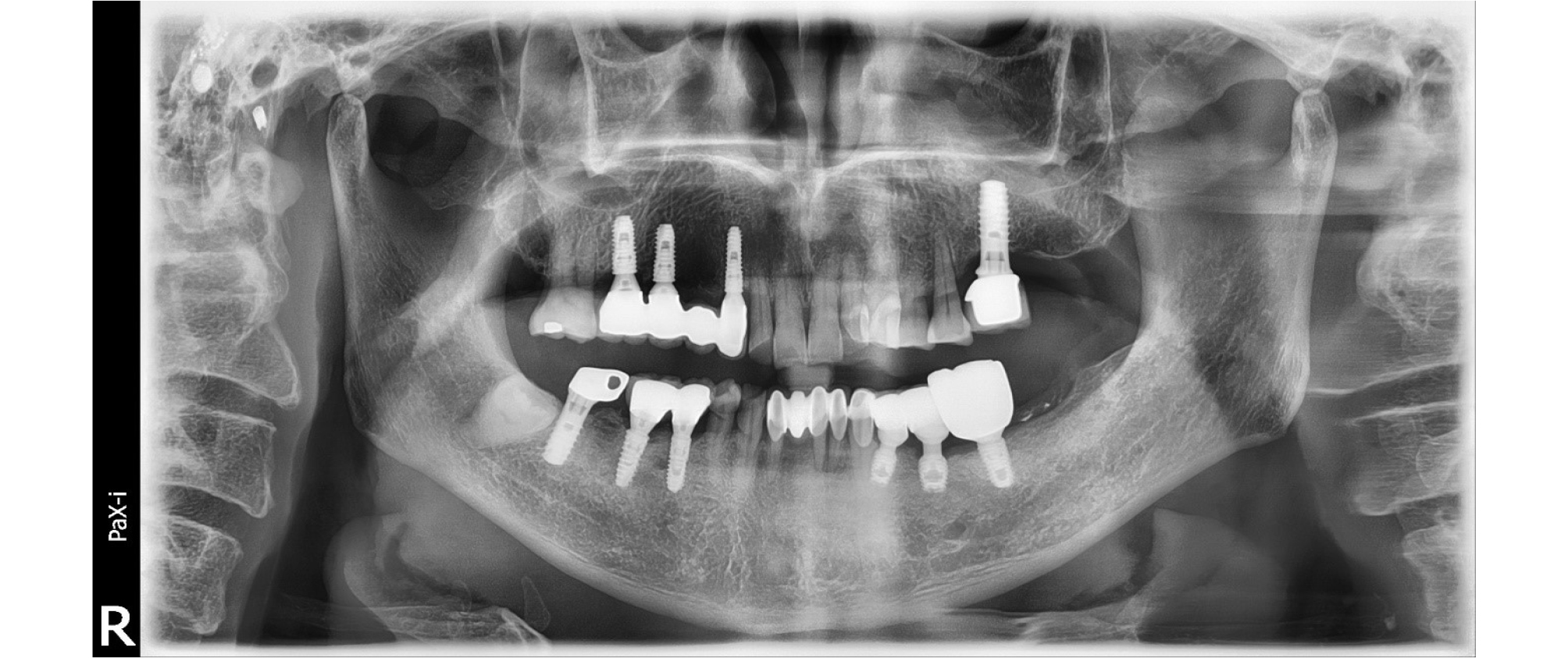
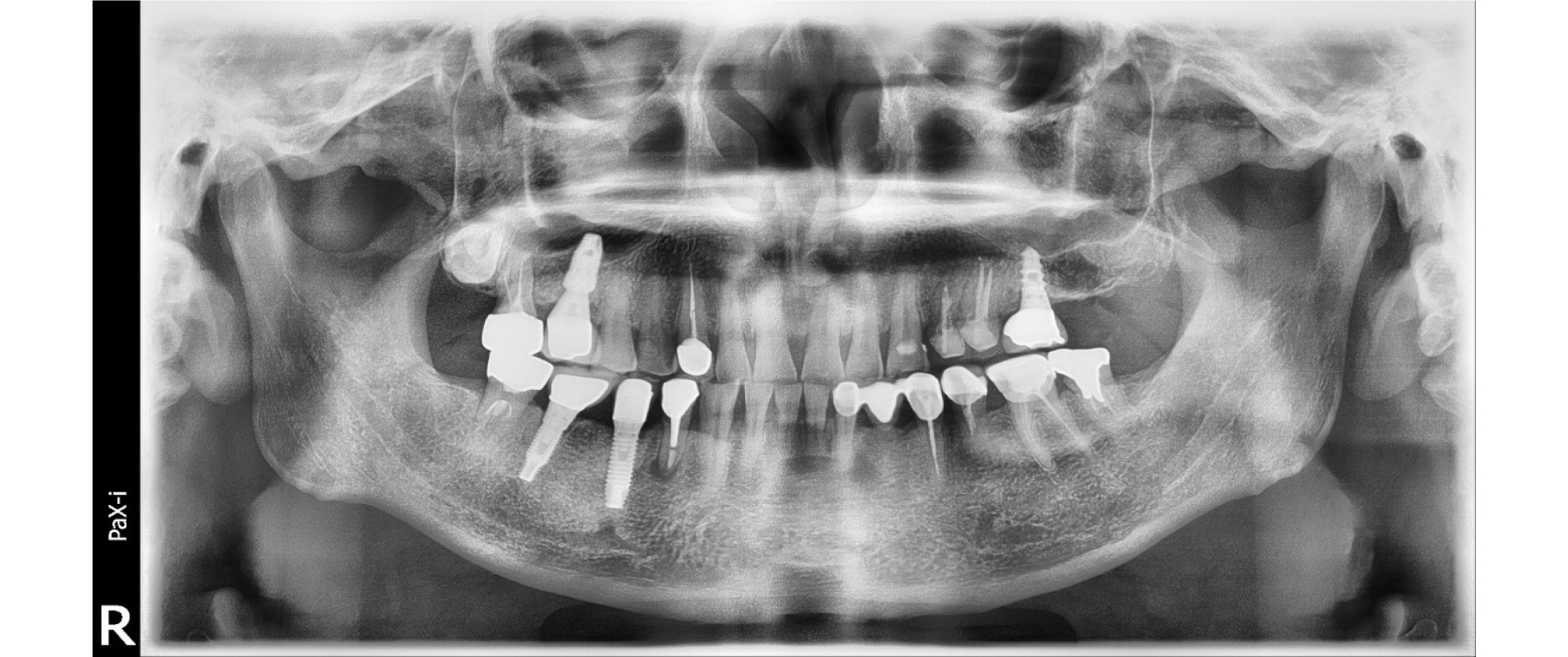
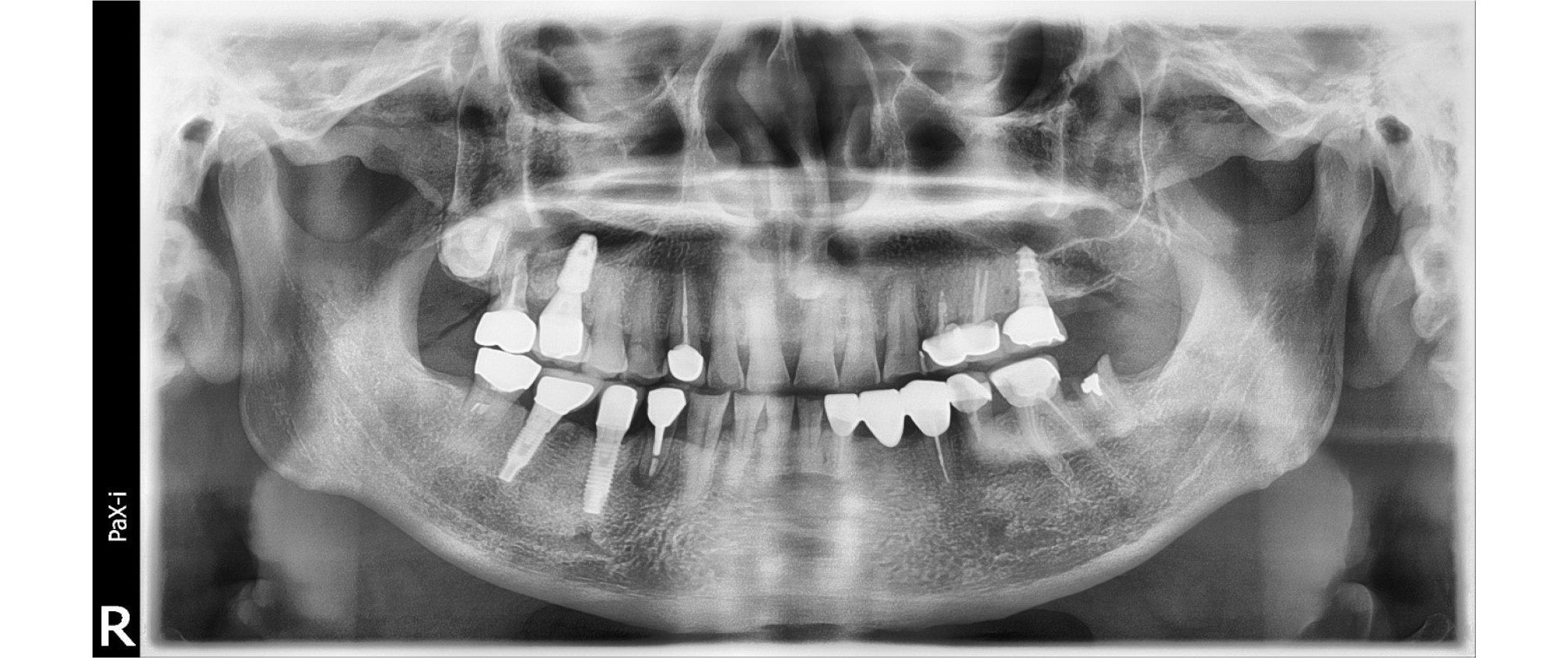
At first visit, we performed non-surgical therapy including scaling, and prescription of antibiotics and anti-inflammatory drugs. We scheduled a surgery to treat a peri-implantitis lesion and bone defect due to implant loss of #36 2 weeks after initial therapy. After the local anesthesia with 2% lidocaine HCL with 1:100,000 epinephrine, intra-sulcular incision was made at the surgical site with blade and interdental knife (Hu-Friedy, Chicago, IL, USA). Mechanical debridement with air-abrasive system (Prophyflex3, KaVo Dental, Biberach, Germany), titanium ultrasonic scaler, and adjunctive chemical decontamination with tetracycline (TC) cotton and solution were performed. On the circumferential defect of #i35, bone graft was done with synthetic bone (MBCP, Biomatlante, Vigneux, France). Guided bone regeneration was tried with MBCP and collagen membrane (Lyoplant ® , Braun Aesculap, Tutsingen, Germany) on #36 explantation defect. The surgical site was sutured with 4-0 e-PTFE (Biotex ® , Purgo biologics, Seongnam, South Korea) using modified horizontal mattress and interrupted suture method. A panoramic radiograph was taken immediately after surgery (Fig. 2). 2 weeks after surgery, the stitch out was done. The patient’s chief complaint including pain and pus discharge was resolved. After 6months of bone graft, we performed first stage implant surgery of #36 (Fig. 3). The grafted MBCP material on the lingual defect of #i35 seemed to be normal bone tissue with naked eye on the first stage implant surgery. The PPD was reduced to 2 mm, and radiographic improvement of 5 mm defect fill was observed after 3 years after surgery (Fig. 4).
2. Case 2
A 50-year-old male patient visited the department of periodontology with discomfort in the right posterior mandible. He received implant surgery on right mandibular second premolar 2 years ago. There we could observe PPD deeper than 10 mm with pus discharge, and peri-implant mucosal swelling. On the panoramic and periapical image, we could detect severe circumferential bone loss around #45 implant (#i45). Marginal bone defect was measured via distance of implant fixture-abutment interface (IP) to most coronal point of bone to implant contact (BIC). The deepest bone defect was detected at distolingual side of #i45 showing a depth of 7 mm. Also, apical lesion of #44 treated with post and crown was detected. Because #44 had no specific symptoms and he didn’t want to treat the lesion, we decided to watch the periapical lesion periodically (Fig. 5).
At 3 months after non-surgical treatment, peri-implantitis surgery was provided for the patient because a suppuration recurred on the defect. On incision and flap elevation, #i45 showed severe lingual bone loss. Mechanical debridement with air-abrasive system (Prophyflex3, KaVo Dental, Biberach, Germany), ultrasonic scaler, and adjunctive chemical decontamination with TC cotton and solution was done. After mechanical and chemical decontamination procedure on #i45, xenogenic bone (OCS-B ® , NIBEC, Seoul, Korea) was filled in the circumferential defect. Surgical site was sutured with 4-0 e-PTFE (Biotex ® , Purgo biologics, Seongnam, South Korea) and 5-0 nylon (Monosof ® , Covidien,
Dublin, Ireland) using modified horizontal mattress and interrupted suture method. 2 weeks after surgery, the stitch out was done, and the patient’s discomfort of swelling and pain disappeared. At 1-year follow-up, the PPD was reduced to 5 mm and the operation site remained stable. Radiographically the bone defect decreased to 3 mm (Fig. 6). Bone defect was filled 4mm compared to baseline. Up to 3 years follow-up, panoramic image showed bone fill stably, and apical lesion of #44 remained stable (Fig. 7).
Ⅲ. Results
Clinical and radiographic improvements were observed in follow-up after treatment. Clinical reduction of PPD and radiographic evidence of bone gain was observed in all 2 patients. Changes in PPD showed reductions of 4 mm, 5 mm in case 1 and 2 respectively. Radiographic bone defect depths decreased by 5 mm, 4 mm respectively, which remained for 3 years (Table 1).
Table 1.
Preoperative and postoperative probing pocket depth (PPD) and radiographic bone defect depth
Ⅳ. Discussion
Several studies reported that surgical treatment with regenerative procedure using bone graft showed statistically significant improvements in radiographic bone fill compared to open flap debridement alone.11 However, there are few studies in Korea that have reported long-term effect of bone graft on peri-implantitis defect. The purpose of this case report was to radiographically evaluate the long-term effect of surgical treatment of peri-implantitis defects using regenerative bone graft procedure for more than 3 years. Clinical reduction of probing pocket depth and radiographic evidence of bone gain was observed and maintained in both patients 3 years after surgery.
There were many studies reporting that non-surgical therapy is effective for reducing inflammation of peri-implant tissue for peri-mucositis but not for peri-implantitis lesion.3, 4, 5 There was a tendency to recurrence of the disease because of insufficient implant surface disinfection.5, 6 Decontamination protocols such as mechanical debridement with air-abrasive system, titanium or plastic curette and ultrasonic scaler were effective for removal of biofilm.2, 6 However using only mechanical method often caused incomplete surface disinfection because of instrument accessibility.6 Therefore, adjunctive chemical decontamination agents including citric acid, tetracycline, chlorohexidine, acidified phenolics, and ethylenediaminetetraacetic acid (EDTA) are commonly used to treat inaccessible area of implant.2, 7 Although many mechanical and chemical decontamination methods were tried clinically, no definite gold standard has been identified.13 Tetracycline have been used with bone graft material in regenerative surgical techniques, due to anti-microbial effect, anti-collagenase, and positive effect with bone graft.14, 15 In a review by Javed et al.,16 systemic and local antibiotic applications led to significant reductions of probing pocket depths in a period between one and six year. Moreover, it was shown that the decontamination procedure using tetracycline followed by guided bone regeneration resulted in arrest of inflammation and radiographic bone fill.7, 1415 TC cotton and solution was used in the decontamination process in this study. However, it was not possible to confirm the effect of TC alone in this study. More sophisticatedly designed studies are needed to demonstrate the beneficial effect of TC conditioning alone, excluding the effect of bone graft.
Following decontamination of the implant surface, regenerative techniques have been introduced to regenerate the bone loss due to infection in an attempt to achieve re-osseointegration of implant surface.17 Regenerative procedures have two main objectives: to support the tissue dimensions during the healing process, avoiding recession of the mucosa, and to enhance the chance of obtaining re-osseointegration, using reconstructive and regenerative techniques/materials.5 So far, there doesn’t seem to be much evidence of which surgical method is superior to the other.
Regarding to grafting materials, autogenous, allogenic, synthetic and xenogenic bone replacement materials were often used for augmentation in bone defects with or without collagen membrane. It seemed allogenic and xenogenic grafts might be almost equivalent to autogenous material.18, 19 In the case of this study, synthetic bone and xenogenic bone were used, and it was found to be effective for defect fill regardless of the type of graft materials, which maintained up to 3 years. However, the factors that could affect the outcomes, such as the type of bone defects, the degree of defects, the type of graft materials, and adjacent teeth or implant types were not controlled in this study, so further studies are needed to evaluate the effects of each factor.
In general, regenerative procedure using membrane or bone graft material alone have been shown to be more effective than open flap debridement alone regarding to bone regeneration and re-osseointegration of peri-implantitis defect.1313 Also several studies reported the effects of combined use of membranes and bone graft materials were superior to those using membranes or bone grafts alone and tend to give the best results.13, 20 However, there is a high variability in the amount of bone fill due to different investigation protocols and measurement. In previous studies of surgical augmentative treatment of peri-implantitis, PPD reduction in the range of 0.74 to 5.4 mm and radiographic bone fill of 57% to 93.3%, and vertical defect reduction varied from 0.2 to 3.77 mm was reported.11 Patients in this study showed a 4-5 mm improvement in radiographic bone defect and PPD. However, the results should be carefully interpreted as it has not been confirmed to be a true histological regeneration.
Ⅴ. Conclusion
In this study, we used regenerative surgical technique, using synthetic graft with absorbable membrane or xenograft without membrane, with mechanical and chemical decontamination to the defect showing circumferential bone loss and intrabony defect. Within the limitation of this case report, bone defects were managed effectively by surgical treatment with regenerative bone graft procedure. Further studies with long term follow up and controlled factor should be needed for treatment protocol of peri-implantitis defect.