Ⅰ. Introduction
Over the past several decades, dental implants have become a common option for restoring edentulous ridges. During this period, peri-implant disease has also become widespread, with a prevalence of up to 72% in the dental implant population.1 Peri-implantitis (PI), one of the most common biological complications of dental implants, is a chronic inflammatory disease causing advanced loss of supporting bone.1 Various non-surgical and surgical treatment modalities for PI have been introduced, however no treatment has conclusively resulted in better outcomes or fewer biological complications. 2 In particular, several studies reported that non-surgical treatments for PI exhibited unsuccessful results because of insufficient implant surface disinfection.3, 4 Since PI is caused by the accumulation of pathological bacterial biofilms, disinfection and decontamination of the affected implant surfaces is crucial.5 ,6
Several methods for implant surface disinfection, including mechanical and chemical modalities, are currently used in the clinical setting. Mechanical methods such as titanium or plastic curettes, ultrasonic scalers, and air-powder abrasives are effective for the removal of biofilms. However, using only mechanical methods often causes incomplete surface disinfection due to a lack of instrument accessibility to the affected area.6, 7 Consequently, adjunctive chemical disinfecting agents such as citric acid, tetracycline, chlorhexidine, acidified phenolics, and ethylenediaminetetraacetic acid (EDTA) are commonly used to treat inaccessible implant areas, but no standardized implant disinfection protocols currently exist.8, 9, 10
This report aims to evaluate the use of adjunctive gel-type desiccant agents (GTDAs) combined with mechanical disinfection and decontamination of inaccessible infected implant surfaces, in two separate cases of surgical treatment for PI.
Ⅱ. Case
1. Case 1
A 61-year-old man visited the periodontal department with a complaint of generalized gingival bleeding. The patient had a multi-unit implant-supported bridge on the upper and lower arches (Fig. 1A, 1B). Previously, the patient had experienced no specific general health problems or drug allergies. According to the patient, the dental implants were installed approximately 7–8 years prior to the visit. The implants on the maxillary left lateral incisor and canine showed suppuration with a deep periodontal pocket depth (PPD) of approximately 8–9 mm around the implants, and vertical bone loss was observed radiographically (Fig. 1C). Subsequently, regenerative PI surgery was scheduled. Thirty minutes before surgery, the patient was administered an antibiotic (Netilmicin 50 mg/mL) and analgesic (Diclofenac 90 mg/2 mL) injection. Local anesthetics (2% lidocaine HCL with 1:100000 epinephrine; Yuhan, Seoul, South Korea) were injected at the surgical site. A crestal incision of the maxillary left central incisor pontic area, and an intra-sulcular incision on the maxillary left lateral incisor and canine were made with an Orban knife (Hu-Friedy, Chicago, IL, USA) fitted with #12 and #15 blades. After incision and flap elevation, labial and distal intra-bony implant defects of the maxillary left lateral incisor were observed (Fig. 2A). Mechanical debridement of the implant surface and removal of granulation tissue were performed with an ultrasonic scaler (SONICflex air scaler, KaVo, Biberach, Germany), and Gracey curette (Hu-Friedy, Chicago, IL, USA). Subsequently, we performed chemical disinfection and decontamination with GTDA (HybenX ® , Epien Medical, Saint Paul, MN, USA) due to the implant fixture proximity, and also because the vertical element of the intra-bony defect caused the distal surface of the implant located at the maxillary left lateral incisor to be almost inaccessible to a mechanical instrument (Fig. 2B). 0.5 mL of GTDA was applied carefully to the surface of implant for 30 seconds, avoiding mucosal contact of GTDA, and then washed out with saline. After mechanical debridement, chemical disinfection, and decontamination, xenografts (The graft ® , Purgo biologics, Seongnam, South Korea), mixed with tetracycline (Chong-Kun-Dang, Seoul, South Korea) at a concentration of 50 mg/ mL, were applied to the bone defect (Fig. 2C, 2D, 2E). The surgical site was sutured with a 4-0 e-PTFE (Biotex ® , Purgo biologics, Seongnam, South Korea) using the interrupted suture method and a modified horizontal mattress suture. Postoperative medication including antibiotics (Kymoxin 500mg tid for 5 days), a nonsteroidal anti-inflammatory drug (Loxoprofen 60 mg tid for 5 days), and a gastric protective agent (Almagel 500 mg tid for 5 days) were administered to the patient.
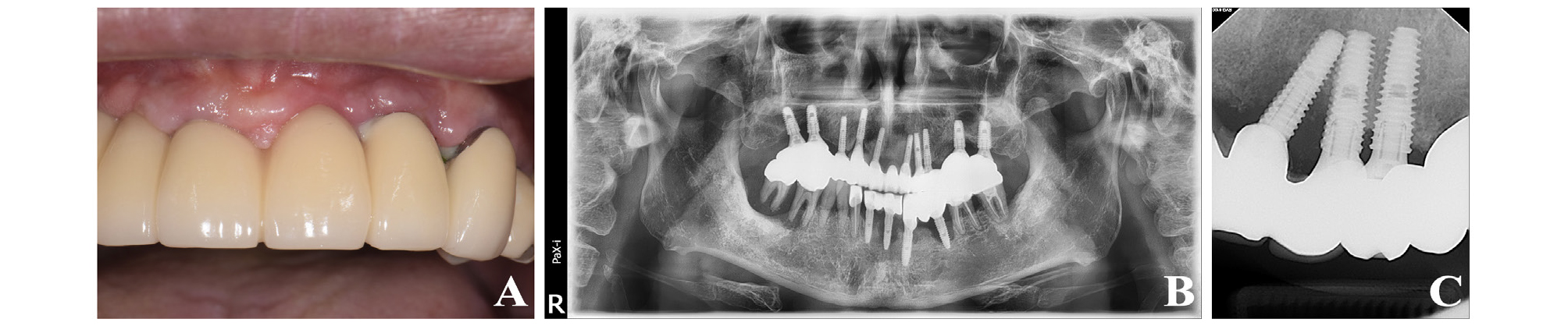
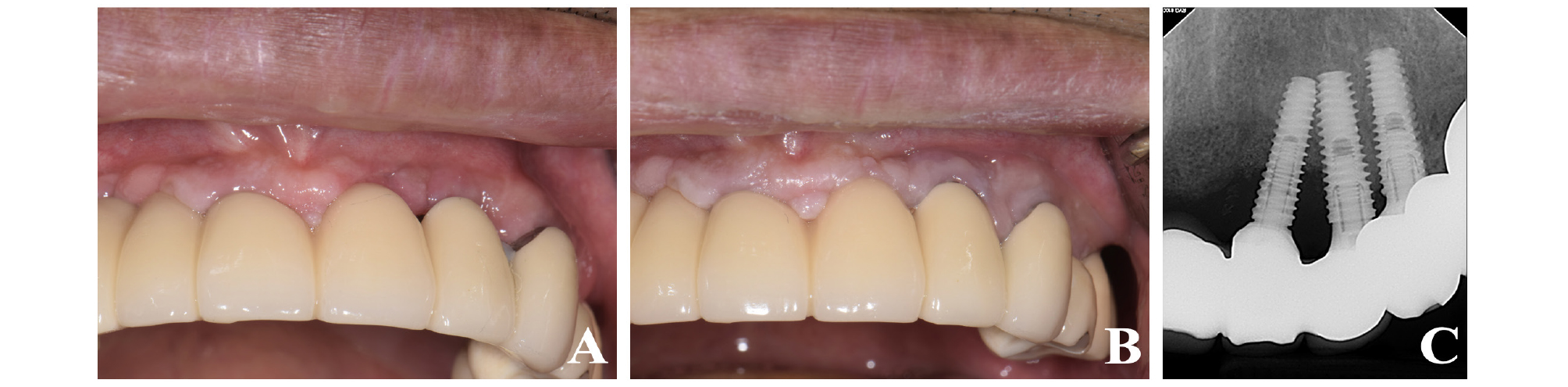
Fig. 1.
Initial intraoral photograph and radiograph of case 1 patient. (A) Gingival swelling and pus discharge are observed in labial sulcus of #22 and #23 implants. (B) Panoramic radiograph. Multiple implants with bone loss were seen. (C) Peri-apical radiograph. Intra-bony defect and implant proximity were observed.
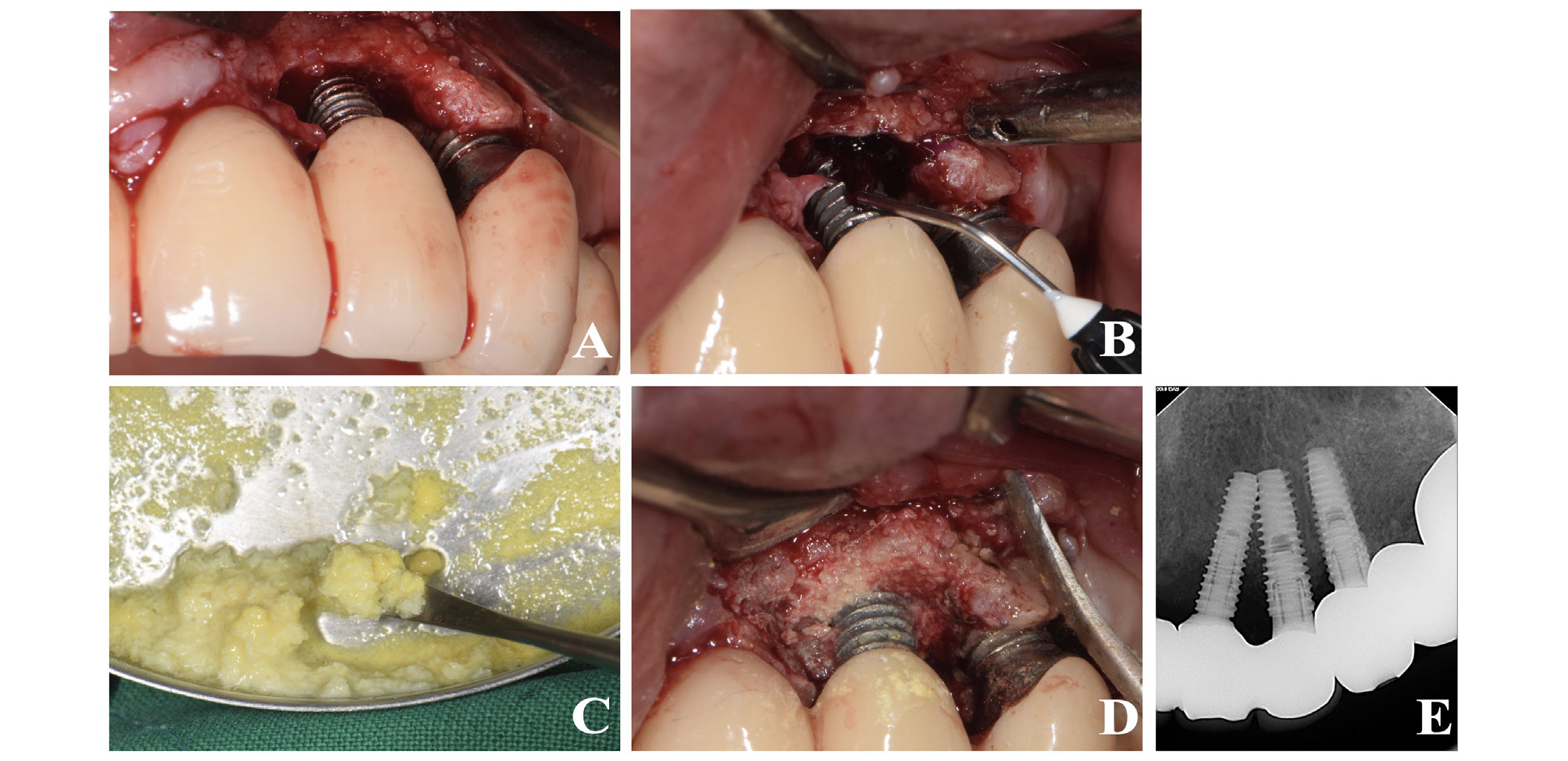
Fig. 2.
Clinical photograph and radiograph of case 1 patient during and after surgery. (A) Deep intrabony defect after flap elevation. (B) Chemical disinfection using gel type desiccating agent (GTDA). (C) Xenografts mixed with tetracycline. (D) Xenogafts mixed with tetracycline were applied in intra-bony defect of #22 and #23 implants. (E) Peri-apical radiograph after surgery.
2. Case 2
A 58-year-old female patient visited the periodontal department. She had previously been informed at a local dental clinic that her mandibular left implants were in poor condition. In addition, the patient also revealed that her maxillary right implants, which were installed approximately 2 years earlier, had been extracted 10 days before the visit. The patient had no specific general health problems or drug allergies. Clinically, the patient had multiple implant-supported restorations at the posterior mandible. The implants at the mandibular left second premolar and first molar showed peri-implant mucosal swelling and bleeding on probing (BOP) (Fig. 4A). A radiographic examination revealed that both implants were installed in very close proximity, and the implants located at the mandibular left second premolar and first molar showed an intra-bony defect (Fig. 4B). Therefore, regenerative PI surgery on the mandibular left second premolar and first molar was scheduled. Thirty minutes before surgery, the patient was administered antibiotic and analgesic injections, and local anesthetics were injected at the surgical site. A sulcular incision from the mid-buccal of the implant located at the mandibular first premolar to the distal portion of the implant located at the first molar was made with an Orban knife fitted with #12 and #15 blades. After incision, the flap was elevated and an intra-bony defect extending from the mesial side of the left mandibular second premolar, to the distal side of the first molar was observed (Fig. 5A). Since the implants were located in very close proximity, mechanical debridement with an ultrasonic scaler and a Gracey curette were followed by GTDA application (Fig. 5B). 0.5 mL of GTDA was applied 30 seconds to the surface of implant while avoiding mucosa contact. After washing of GTDA with saline, xenografts mixed with tetracycline at a concentration of 50 mg/mL were applied to the intra-bony defect (Fig. 5C). The flap was sutured with the interrupted and modified horizontal mattress suture method using a 4-0 e-PTFE. Peri-apical radiographs were taken to confirm the presence of the graft material applied to the intra-bony defect (Fig. 5D). Postoperative medication, including antibiotic and nonsteroidal anti-inflammatory drugs, was administered to the patient.
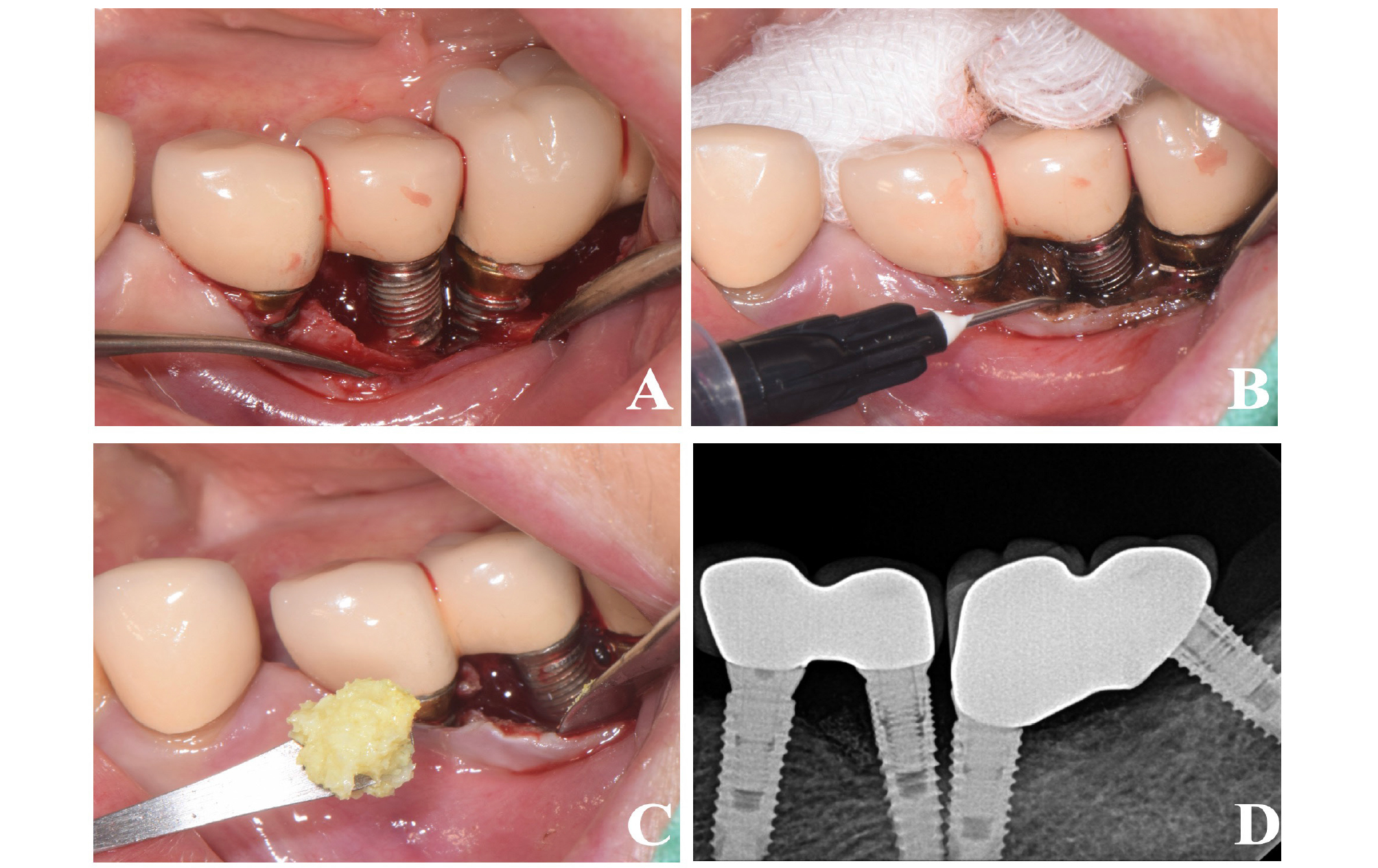
Fig. 5.
Clinical photographs and radiograph showing surgical procedures of case 2 patient. (A) Intrabony defect and implant proximity after flap elevation. (B) Chemical disinfection using GTDA. (C) Xenografts mixed with tetracycline application after implant surface disinfection. (D) Peri-apical radiograph after surgery.
Ⅲ. Results
1. Case 1
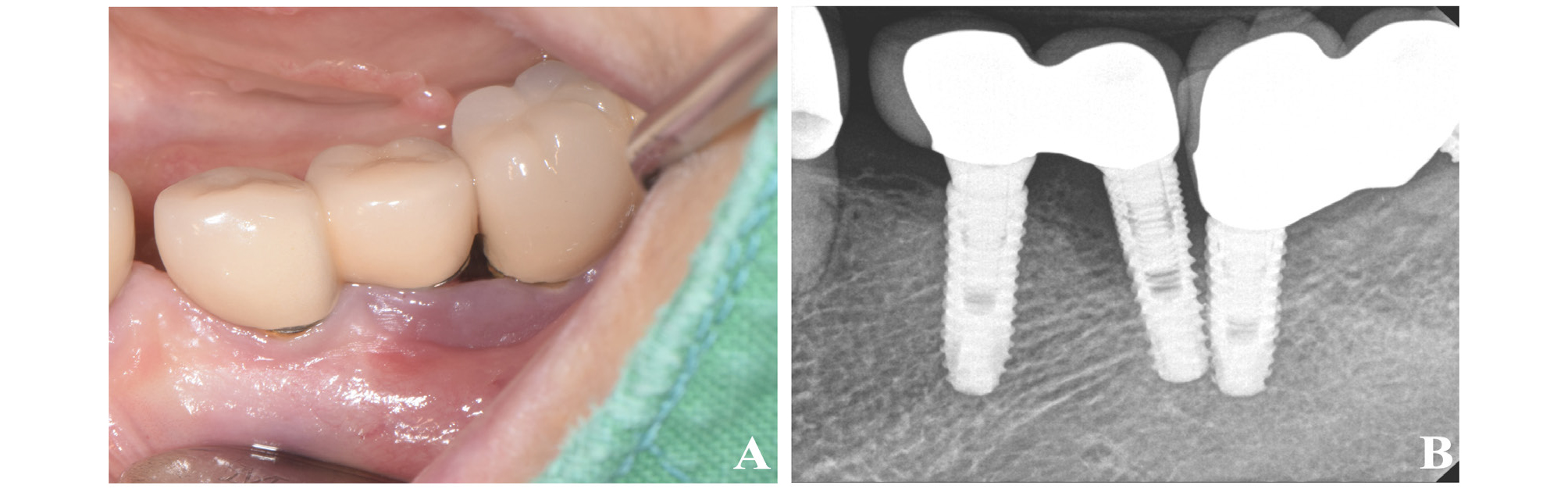
Two weeks after surgery, a stitch out was performed, and reductions in gingival swelling and pus discharge were observed (Fig. 3A). Patient did not report any post-operative complications at two weeks after surgery. During the 6-month follow-up, uneventful healing, improved clinical results with a reduced PPD of approximately 2–3 mm, and resolution of BOP as well as suppuration were maintained (Fig. 3B). Additionally, the xenograft applied to the surgical site was still observed on the periapical radiograph (Fig. 3C).
2. Case 2
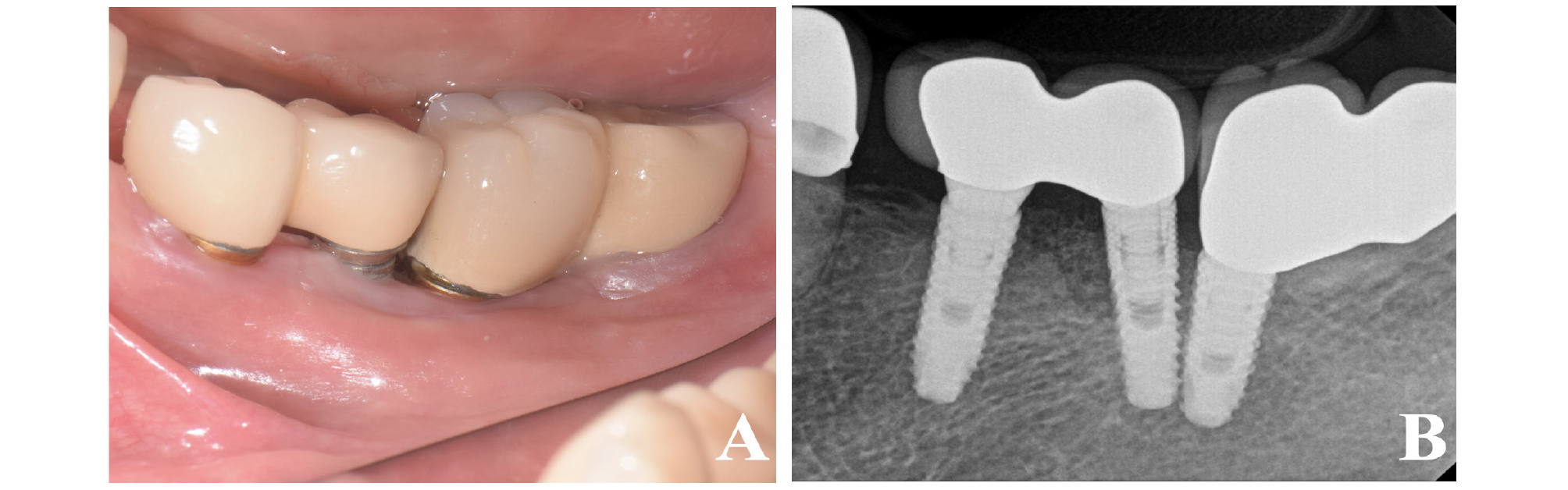
The stitch out was performed 2 weeks after surgery. Patient also did not report any post-operative complications at 2 weeks after surgery. At the 6-month follow-up examination, uneventful healing with resolution of BOP was observed, and the PPD around the implant was reduced from 8–9 mm to 4 mm (Fig. 6A). The periapical radiograph confirmed the presence of the xenograft applied to the intra-bony defect, and revealed a stable bone level (Fig. 6B).
Ⅳ. Discussion
In this report, we describe two cases of implant surface disinfection using GTDA, followed by bone grafting. In both cases, the clinician faced difficulties accessing the implant surface due to the implant insertion path and location. In case 1, a deep intra-bony defect causing limited sight and instrumentation was present around the implant located at the left maxillary lateral incisor. In case 2, implants at the left mandibular second premolar and first molar were too close to each other to allow curette or ultrasonic device application. Because of these difficulties, mechanical debridement alone was not sufficient to disinfect contaminated implant surfaces.
Non-surgical treatment of PI has shown only modest efficacy, while surgical treatment has provided more predictable results.2, 11 Non-surgical treatment with mechanical debridement and local administration of antibiotics has demonstrated a significant reduction of BOP, but other clinical parameters such as PPD and bone levels remain, suggesting probable PI recurrence.12 Several surgical treatment procedures including resective and regenerative surgical techniques have been introduced, but evidence of the superiority of either method is lacking.2 Clinicians may choose various surgical methods depending on the defect morphologies, and for intra-bony defects in particular, regenerative surgical techniques provide improved results in PPD reduction and radiographic bone filling.11
In our study, since both cases showed intra-bony defects, we employed a regenerative surgical technique using xenograft bone particles without a membrane. A systematic review and meta-analysis found that bone grafting without a membrane around a PI affected implant demonstrated a 2.32 mm mean PPD reduction, and a 2.10 mm mean radiographic bone fill.11 A previously published randomized controlled clinical trial reported that the additional use of membrane with bone graft material resulted in no significant differences in PPD reduction and radiographic bone fill in up to 5 years of follow-up examinations.13 In the current cases, we used a Gracey curette and ultrasonic scaler tip for mechanical decontamination during PI surgical treatment. These mechanical methods have provided different levels of accessibility depending on implant thread and valley, but the methods demonstrated a similar cleaning efficacy of 74.70% and 66.95% of the uncleaned surface, respectively.6, 14
Due to the limitations of mechanical debridement, chemical decontamination methods are commonly used. We used tetracycline and GTDA as decontaminating agents in the current cases. Tetracycline, used with bone graft material, is known to prevent bone loss and increase bone formation by increase collagen type 1 synthesis.15 GTDA is chemical desiccant, consisting of 60% sulfonated phenolics, 28% sulfuric acid, and 12% water.8 The sulfonic/sulfuric acids in GTDA have a strong affinity for water, which constructs the aqueous polysaccharide gel structure in biofilms. Consequently, GTDA disrupts the biofilm on the infected implant surface, causing molecular denaturation and tissue coagulation in the superficial layer of supporting tissue. This rapid and irreversible desiccation mechanism is believed to enhance the effect of mechanical debridement.16, 17 We deciced to use additional GTDA because Gel-type carrier of GTDA is easy to apply to implant surface and safe for gingiva and teeth since GTDA targets only damaged soft tissue and less erosive than citric acid or lactic acid.
Since bacterial biofilms and the periodontal pathogens within them are the main causes of PI, it is crucial to remove biofilms on implant surfaces. Application of GTDA during mechanical debridement can enhance the efficacy of instrumentation. This effect was reported in a recent study combining GTDA with non-surgical treatment, resulting in BOP improvement, reduced total bacterial load, and inflammatory cytokine reduction.7, 147,14 Notably, the adjunctive use of GTDA demonstrated a prolonged and significant reduction of orange and red complex bacteria, compared to non-surgical treatment only.18
We have found that GTDA can safely be used adjunctively to decontaminate implant surfaces for surgical PI treatment, including regenerative surgical techniques. In our cases 1 and 2, patients showed no discomfort and healed uneventfully with no recurrence of inflammation throughout the follow-up period. This product may easily be used for treatment of PI with various modalities in different clinical circumstances, and it is particularly well suited for poorly accessible implant surfaces. Nevertheless, further longitudinal and interventional studies are required to determine the ultimate efficacy and safety of GTDA.