Ⅰ. Introduction
Ⅱ. Materials and Methods
1. Study Design and Patient Selection
2. Surgical and Prosthetic Procedures
3. Outcome Measurements
Ⅲ. Results
1. Implant Survivals and Failures
2. Radiographic Assessment
Ⅳ. Discussion
Ⅴ. Conclusion
Ⅰ. Introduction
Pneumatization of the maxillary sinus and alveolar bone loss after tooth extraction make implant placement in the maxillary posterior area challenging. Sinus floor elevation (SFE) is a highly effective procedure that ensures bone height for implant placement in maxillary molars.
Sinus floor elevation is usually performed using the lateral window and transcrestal approaches. There are various studies on the indications for both procedures. Summer proposed performing SFE using an osteotome via a transcrestal approach, with a residual alveolar bone height of at least 6 mm.1 In comparison to the lateral window approach, the transcrestal approach has the advantage of being minimally invasive with less time, potentially reducing patient discomfort after surgery.
In the osteotome SFE(OSFE) procedure, access to the maxillary sinus membrane is achieved via the crestal approach using a sinus osteotome. Several recent studies have shown predictable outcomes, even with limited residual bone height (RBH, <6 mm).2,3,4 The OSFE procedure can be performed with or without bone grafting. Several studies have suggested performing bone grafting during the OSFE procedure,3, 5,6,7 while others have suggested that bone grafting may not be necessary.2,8,9,10,11 Therefore, the use of bone-graft materials in OSFE remains controversial.
Most studies have assessed the outcomes of OSFE using implant survival rates and bone gain; however, the factors affecting the outcomes of this procedure remain unclear. Several studies have suggested that RBH is the most important factor affecting the selection of treatment options. However, it has not been clearly stated which SFE procedure should be selected according to the height of the residual bone.
This study retrospectively evaluated the outcomes of the OSFE procedure without bone grafting by analyzing endosinus bone gain (ESBG) in the maxillary sinus according to RBH and implant protrusion length (IPL).
Ⅱ. Materials and Methods
1. Study Design and Patient Selection
First, this was a retrospective study. The study protocol was approved by the Institutional Review Board (IRB) of the Wonkwang University Daejeon Dental Hospital (IRB No. W2304/022-001). Between May 2015 and September 2022, 36 patients were enrolled in the study at the Department of Oral and Maxillofacial Surgery, Wonkwang University Daejeon Dental Hospital.
Patients were selected according to the following inclusion criteria: (1) patients in need of implant treatment in the posterior maxilla; (2) the OSFE procedure was performed without bone grafting; (3) primary stability of implants had to be achieved; and (4) the follow-up period after surgery was at least six months. Exclusion criteria were as follows: (1) patients with bone-added OSFE procedure; and (2) history of previous implant placement, or bone augmentation at the operative site.
A total of 44 implants were placed, all of which had SLA (sandblasting large grit and acid etching) surface treatment (Osstem TSIII SA®; Osstem implant system, Busan, Korea/Dentium superline®; Dentium implant system, Seoul, Korea/ITI Roxolid SLActive®; Institut Straumann AG, Basel, Switzerland). Among the 44 implants, 8 and 36 were in the premolar and molar areas, respectively. All the implants were placed using the OSFE procedure without the bone graft material. The distribution of patients and implants, classified based on an RBH of 5 mm on panoramic radiographs obtained immediately after implant surgery (P1), are shown in Table 1.
Table 1.
Overview of patients and implant data
| ¹RBH at P1<5 mm (N=22) |
RBH at P1≥5 mm (N=22) |
Total (N=44) | p-value | |
| Gender | .223 | |||
| Male | 7 (31.8%) | 12 (54.5%) | 19 (42.2%) | |
| Female | 15 (68.2%) | 10 (45.5%) | 25 (56.8%) | |
| Age | 56.7 ± 14.8 | 57.3 ± 13.9 | 56.7 ± 14.5 | .884 |
| Implant type | .493 | |||
| Osstem | 18 (81.8%) | 15 (68.2%) | 33 (75%) | |
| Dentium | 3 (13.6%) | 4 (18.2%) | 7 (15.9%) | |
| Straumann | 1 (4.5%) | 3 (13.6%) | 4 (9.1%) | |
| Implant location | 1.000 | |||
| Premolar | 4 (18.2%) | 4 (18.2%) | 8 (18.2%) | |
| Molar | 18 (81.8%) | 18 (81.8%) | 36 (81.8%) | |
| Follow-up period | 24.8 ± 23.7 | 24.3 ± 15.4 | 24.7 ± 20.2 | .928 |
| Implant length | 1.000 | |||
| 8.5 | 7 (31.8 %) | 6 (27.3%) | 13 (29.5%) | |
| 10 | 15 (68.2%) | 16 (72.7%) | 31 (70.5%) | |
| Implant width | .464 | |||
| ≤4.5 mm | 21 (95.5%) | 21 (95.5%) | 42 (95.5%) | |
| >4.5 mm | 1 (4.5%) | 1 (4.5%) | 2 (4.5%) | |
| Bone density | .469 | |||
| D2 | 0 (0.0%) | 2 (9.1%) | 2 (4.5%) | |
| D3 | 22 (100.0%) | 20 (90.9%) | 42 (95.5%) | |
| RBH at P1 | 4.1 ± 0.9 | 6.1 ± 0.7 | 5.0 ± 1.3 | <.001** |
| Implant protrusion length | 5.6 ± 1.3 | 3.8 ± 1.1 | 4.8 ± 1.5 | <.001** |
| *Significant p-value <.05 | ||||
2. Surgical and Prosthetic Procedures
All the patients received intramuscular injections of antibiotics (Gentacin Injection®; Humedix, Sungnam, Korea) and anti-inflammatory analgesics (Dicknol injection®; Myung Moon Pharm, Seoul, Korea) before the surgery.
The surgical site was locally anesthetized with 2% lidocaine containing 1:100,000 epinephrine. A full-thickness flap was raised by making mid-crestal and vertical incisions. Initial drilling was performed at approximately 1 mm less than the premeasured RBH. Sequential drilling was performed from a 2.0 mm pilot drill to a 4.0 mm twist drill while checking the bone quality. After performing an osteotomy on the remaining cortical bone at the maxillary sinus floor by malleating with a 2.5-mm diameter Summers osteotome, the maxillary sinus floor membrane was carefully elevated. After securing the space for implant placement, the hole for implant placement was sequentially enlarged using 3.0- and 4.2-mm diameter osteotomes.
Sinus membrane perforation was visually checked using a mirror. The sinus floor was then carefully lifted using a depth gauge to check the integrity of the Schneiderian membrane integrity. If maxillary mucosal perforation was confirmed, repair was performed using collagen membrane and fibrin glue (Tisseel®; Baxter AG, Vienna, Austria).
The fixture was installed at a speed of <60 rpm, and a torque wrench was used to check the installation torque for a final 1 mm. All the implants were submerged after the cover screws were fastened. Interrupted sutures were applied using 3-0 non-absorbent suture (Biotex®; Purgo Biologics, Sungnam, Korea). Antibiotics and anti-inflammatory medications were administered orally for three days after the surgery. A second surgery was performed 4–6 months later.
3. Outcome Measurements
3.1. Implant survival
Survival criteria were established using the procedure proposed by Buser12 and Cochran13: (1) absence of clinically detectable implant mobility; (2) absence of pain or any subjective sensation; (3) absence of recurrent peri-implant infection; and (4) absence of continuous radiolucency around the implant.
Implant failure was considered in cases of implant loss, mobility, removal due to progressive marginal bone loss, severe peri-implant infection, or implant fracture. Peri-implant infection was defined as a deep peri-implant periodontal pocket (>5 mm) with bleeding or pus upon probing.14
3.2. Radiographic analysis
Panoramic imaging was performed for each patient before the surgery (P0), immediately after the implant surgery (P1), six months after the surgery (P2), one year after the surgery (P3), and at the final follow-up (F). Radiographic measurement was performed using INFINITT (Infinitt Healthcare®; Seoul, Korea) software by one examiner not participating in the surgical procedure. Radiographs were analyzed at P1, P2, P3, and F. The following parameters were measured on both the mesial and distal sides of the implant using ImageJ program® (National Institutes of Health, Bethesda, USA).
Fig. 1 shows the landmarks used for the radiographic analysis: (a) implant longitudinal axis; (b) implant collar line: the most coronal level of the implant thread vertical to (a); (c) implant apex line: the most apical level of the implant vertical to (a); (d) sinus floor cortical line: a line along with the sinus floor cortical bone; (e) RBH at the medial (RBHm, ) or distal (RBHd, ): distance from (b) to (d) parallel to (a), always positive, and only measured at the baseline; (f) IPL at the medial (IPLm, ) or distal (IPLd, ): distance from (d) to (c) parallel to (a), always positive, and only measured at the baseline.
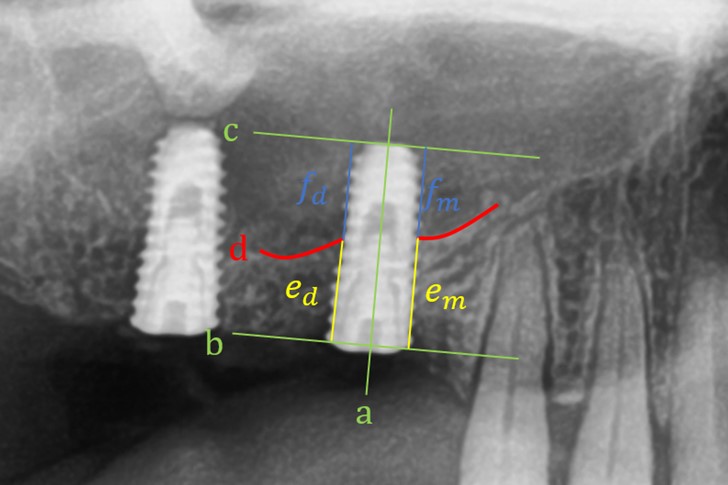
Fig. 1.
Landmarks used for the radiographic analysis.
a Implant longitudinal axis
b Implant collar line: the most coronal level of the implant thread vertical to ‘a’
c Implant apex line: most apical level of the implant, vertical to ‘a’
d Sinus floor cortical line: a line along with the sinus floor cortical bone
e RBH: distance from (b) to (d), parallel to (a)
f IPL: distance from (d) to (F), parallel to (a)
(RBH, residual bone height; ESBG, endosinus bone gain)
The radiographic parameters were evaluated as follows: (1) Implant length (IL): distance from (b) to (c), parallel to (a), and always positive; (2) RBH: the mean of RBHm () and RBHd (); and (3) IPL: the mean IPLm () and IPLd ().
3.3. Statistical analysis
Statistical analysis was performed using R Studio® 2023.03.0+386 (JJ Allaire, Boston, USA). The threshold for statistical significance was set at (p < .05). Independent factors included sex, age, implant type, membrane perforation, implant location (premolar or molar), implant length, implant width, bone density, initial stability, RBH classification (RBH ≥5 mm or RBH <5 mm), and IPL classification (IPL ≥4 mm or IPL <4 mm). The Pearson’s correlation analysis was used to compare the relationship between RBH and ESBG, IPL and ESBG, and membrane perforation and ESBG at the final follow-up. The Welch’s two-sample t-test was applied to compare the ESBG between the RBH groups (RBH <5 or ≥5 mm) and IPL classification (IPL <4 or ≥4 mm).
Ⅲ. Results
1. Implant Survivals and Failures
Membrane perforation was recorded in five cases at the time of implant placement. Initial stability was observed in 28 (63.6%) patients with ≥35 N, three (6.8%) with 26–35 N, seven (15.9%) with 16–25 N, two (4.5%) with 10–15 N, and four (9.1%) with ≤10 N. At the final follow-up, all implants were clinically stable. The final follow-up (F) period averaged 24.7 months, ranging between 17–77 months. The distribution of implants according to implant survival, ESBG, membrane perforation, and initial stability based on an RBH of 5 mm on panoramic radiographs taken immediately after implant surgery (P1) is presented in Table 2.
Table 2.
Distribution of implants according to implant survivals, membrane perforation, and initial stability based on RBH of 5 mm at P1
| ¹RBH at P1 <5 mm (N=22) |
RBH at P1 ≥5 mm (N=22) |
Total (N=44) | p-value | |
| Implant survival | ||||
| 22 (100%) | 22 (100%) | 44 (100%) | ||
| Membrane perforation | 1.000 | |||
| Non-existence | 19 (86.4%) | 20 (90.9%) | 39 (88.6%) | |
| Existence | 3 (13.6%) | 2 (9.1%) | 5 (11.4%) | |
| Initial stability | .234 | |||
| 35 N | 11 (50.0%) | 17 (77.3%) | 28 (63.6%) | |
| 26–35 N | 3 (13.6%) | 0 (0.0%) | 3 (6.8%) | |
| 16–25 N | 5 (22.7%) | 2 (9.1%) | 7 (15.9%) | |
| 10–15 N | 1 (4.5%) | 1 (4.5%) | 2 (4.5%) | |
| Less than 10N | 2 (9.1%) | 2 (9.1%) | 4 (9.1%) | |
| *Significant p-value <.05 | ||||
2. Radiographic Assessment
The RBH measured at P1 was between 1.92–7.28 mm, with an average value of 5.04 ± 1.31 mm. The IPL was between 1.16–8.20 mm, with an average value of 4.77 ± 1.47 mm at P1. The average ESBG progressively increased from 1.68 mm at P2 to 2.11 mm at P3 and 2.21 mm at F examination. The changes in each value over time are in Table 3.
Table 3.
Summary of radiographic parameters at each time point
| ¹RBH (mm) | 2IPL (mm) | 3ESBG (mm) | |
| At implant placement (P1) | 5.04 ± 1.31 | 4.77 ± 1.47 | |
| Six months (P2) | 6.72 ± 1.59 | 3.09 ± 1.66 | 1.68 ± 1.29 |
| One year (P3) | 7.15 ± 1.69 | 2.66 ± 1.80 | 2.11 ± 1.07 |
| Final follow-up (F) | 7.25 ± 1.69 | 2.56 ± 1.28 | 2.21 ± 1.24 |
2.1. Relationship between RBH and ESBG
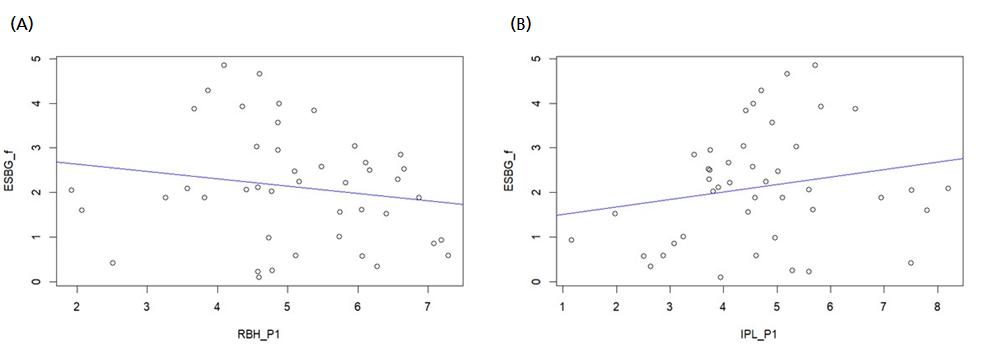
The correlation analysis results between the RBH measured at P1 and ESBG values at P2, P3, and F were not statistically significant (p = .8, .25, and .28, respectively). This indicates that there was no statistically significant relationship between RBH at P1 and ESBG. A simple linear regression graph of RBH at P1 and ESBG at F is shown in Fig. 2A (p = .2677).
The RBH group was divided based on an RBH of 5 mm at P1. There were 22 implants in each group, with mean RBH values of 4.06 ± 0.90 and 6.02 ± 0.81 mm, respectively. Significant differences in ESBG were observed between the two groups at P2 and P3 (p = .019 and .019, respectively), but not at F (p = .15). These findings indicate that the RBH <5 mm group had substantial bone gain of up to one year after the surgery compared to the RBH ≥5 mm group; however, there was no significant difference between the two groups at the final follow-up period. Details of the changes in ESBG based on an RBH of 5 mm are listed in Table 4.
Table 4.
Endosinus bone gain at P2, P3, and final follow-up according to RBH
| ¹RBH (mm) | |||
| <5 (N=22) | ≥5 (N=22) | p-value | |
| 2ESBG at P2 | 2.09 ± 1.43 | 1.26 ± 0.95 | .019 ** |
| ESBG at P3 | 2.61 ± 1.29 | 1.77 ± 0.66 | .019 ** |
| ESBG at final follow-up | 2.40 ± 1.48 | 2.01 ± 0.87 | .15 |
| *Significant p-value <.05 | |||
2.2. Relationship between IPL and ESBG
The correlation analysis results between the IPL measured at P1 and ESBG values at P2, P3, and F were not statistically significant (p = .17, .32, and .19, respectively). This indicates that there was no significant correlation between the IPL and ESBG. A simple linear regression graph of the IPL at P1 and ESBG at F is shown in Fig. 2B (p = .1947).
The IPL group was divided based on an IPL of 4 mm at P1. Significant differences in the ESBG were observed between the two groups at P2 and F (p = .038 and .027, respectively); however, no difference in ESBG was found at P3 (p = .173). These outcomes indicate that the IPL ≥4 mm group had substantial bone gain at six months after the surgery and at the final follow-up period compared to the IPL <4 mm group; however, there was no significant difference between the two groups at one year after the surgery. Details of the changes in ESBG based on an IPL of 4 mm are listed in Table 5.
Table 5.
Endosinus bone gain at P2, P3, and final follow-up according to IPL
| ¹IPL (mm) | |||
| <4 (N=15) | ≥4 (N=29) | p-value | |
| 2ESBG at P2 | 1.11 ± 1.00 | 1.90 ± 1.32 | .038 ** |
| ESBG at P3 | 1.89 ± 0.76 | 2.21 ± 1.17 | .173 |
| ESBG at final follow-up | 1.72 ± 0.93 | 2.40 ± 1.29 | .027 ** |
| *Significant p-value <.05 | |||
2.3. Relationship between membrane perforation and ESBG
The following table presents the differences in RBH and ESBG at P2, P3, and F based on membrane perforation during the SFE (Table 6). The RBH at P1 was significantly higher in the non-perforation group (5.2 ± 1.2 mm) compared to the perforation group (4.0 ± 1.9 mm) (p = .046). These findings indicate that membrane perforation occurs more frequently during surgery in patients with limited RBH. However, there was no significant difference in the ESBG at P2, P3, and F based on membrane perforation.
Table 6.
Residual bone height and ESBG at P2, P3, and final follow-up according to membrane perforation
| Membrane perforation | |||
| Non-existence (N=39) | Existence (N=5) | p-value | |
| ¹RBH at P2 | 5.2 ± 1.2 | 4.0 ± 1.9 | .046 |
| RBH at P3 | 6.9 ± 1.3 | 5.5 ± 2.7 | .328 |
| RBH at final follow-up | 7.3 ± 1.5 | 6.3 ± 2.4 | .214 |
| 2ESBG at P2 | 1.7 ± 1.3 | 1.5 ± 1.5 | .851 |
| ESBG at P3 | 2.0 ± 1.1 | 2.1 ± 1.0 | .864 |
| ESBG at final follow-up | 2.1 ± 1.3 | 2.3 ± 1.0 | .687 |
| *Significant p-value <.05 | |||
Ⅳ. Discussion
In this study, the implant survival rate and radiological bone remodeling were evaluated for 44 implants in 36 patients who underwent OSFE without bone grafting, with follow-up periods between 6–78 months. The implant survival rate in this study was 100%, which is higher than that in most studies and comparable to the outcomes of several other studies.2,8,9 Notably, none of the implants with an RBH of <5 mm failed, possibly because of the limited sample size and more delicate handling used in these cases.
Other studies have indicated a predictable result when the RBH was >5 mm. For example, Pjetursson15 reported an implant survival rate of 90% in areas with an RBH of <5 mm compared to 100% in areas with an RBH of >5 mm. A recent meta-analysis found that OSFE may affect the success of implants placed in areas with an RBH of <4 mm. According to Calin,16 even if new bone formation occurs around the extruded implant immediately after membrane elevation, the initial residual bone remains crucial for implant stabilization.
However, numerous studies contradict this hypothesis. Some authors have performed OSFE without bone grafting in areas with an RBH of <4 mm and reported a high implant survival rate.10,17 In this study, the sites of implant placement were divided into two groups (RBH ≥5 and <5 mm), and the implant survival rate in both groups was 100%. However, when comparing ESBG, bone gain was higher in the group with an RBH of <5 mm, which is consistent with the results of previous studies. ESBG was significantly higher in the group with RBH <5 mm than in the group with RBH ≥5 mm up to 1 year after surgery, but there was no significant difference at the last follow-up. This result indicates that performing OSFE without bone grafting in patients with RBH less than 5 mm can achieve a high implant survival rate and predictable outcomes. However, because the follow-up period in this study varied from 6 months to 6.5 years, further investigation is required.
Endosinus bone gain is a common measurement method used to evaluate the outcomes of the SFE procedure, and all implants in this study achieved ESBG without the use of bone graft materials. During the observation period, the mean ESBG was 2.21 ± 1.22 mm and remained stable. These results indicate that the new bone remains quantitatively and qualitatively stable and durable over time under normal masticatory loading.
Other previous studies have analyzed potential factors contributing to ESBG and found a positive correlation between final ESBG and initial IPL measured immediately after surgery.4,18 In this study, the significance test between the initial IPL and ESBG at P2, P3, and F did not show significant results. However, when the initial IPL was divided into two groups at 4 mm, the ESBG at P2 and F showed significant results. The ESBG was significantly higher in the group with IPL of ≥4 mm compared to that in the group with IPL of <4 mm at six months postoperatively and at the final follow-up period, which corresponds to previous studies.
The most common complication during SFE was sinus membrane perforation, which occurred in 8.8% (5/44) of the SFE sites in this study, a higher rate than that reported in other studies. In 2008, Tan et al. reported a perforation rate of 3.8% in 1,776 SFE sites, and another systematic review by Del Fabbro et al. in 2012 reported a rate of 4.2% in 3,131 SFE sites. It was suggested that the effect of sinus membrane perforation in SFE surgery on implant survival rate was not statistically significant.19,20 Similarly, in this study, none of the five implants placed at sites with sinus membrane perforation failed, indicating that sinus membrane perforation had no significant effect on the survival rate of the implant.
In this study, panoramic images were used to evaluate endosinus bone remodeling. One of the major limitations of this study is the likelihood of radiographic image overlap and distortion. Additionally, owing to the retrospective study design, the number of patients in each group was not equal, and the sample size of some groups was relatively limited. Therefore, studies with a larger sample size and better long-term outcomes are required.
Ⅴ. Conclusion
Despite the limitations of this study, the findings suggest that OSFE procedure without bone grafting is a predictable treatment method for maxillary SFE in terms of bone regeneration and implant survival, even when RBH is limited to ≤5 mm. The ESBG was significantly higher in the group with RBH of <5 mm compared to that in the group with RBH of ≥5 mm for up to one year after implant placement. All 22 implants placed where the RBH was <5 mm demonstrated 100% survival and success rates after an average follow-up period of two years.