Ⅰ. Introduction
Introduced in the late 1970s, delayed implant placement, which involves placing implants on a healed extraction site, is now widely known as a stable technique with a good prognosis.1 According to Hammerle et al.,2 after tooth extraction, the implant placement delay time can be classified as Type I, II, III, or IV. Immediate placement, which involves placing the implant on a fresh extraction site, is considered Type I, and the conventional implant placement method, delayed placement, is Type IV.
The immediate implant placement method is advantageous in that it reduces the number of surgeries a patient undergoes and shortens the length of the treatment period. For anterior dentition in particular, immediate restoration maintains better aesthetics than delayed implant placement, however, there are disadvantages that should be considered. These disadvantages include: the possibility that implants may be placed without pathological issues being healed after extraction, a lack of keratinized gingiva, and the inability to completely seal the soft tissue after implant placement.3 Depending on the detailed treatment method, however, immediate placement can have advantages, such as the reduction of soft and hard tissue resorption, and the selection of the optimal position for the implant. Immediate placement of an implant on the posterior dentition also has a number of advantages, and is being occurring more frequently.4
The mandibular first molar is the first permanent tooth to erupt, and has a clinically important position in terms of the maintenance of both the arch formation and occlusion, along with a core function during mastication. Loss of the mandibular first molars causes non-physiological occlusion and tipping of adjacent teeth, resulting in extrusion of the antagonistic teeth.5 After extraction, notable changes occur at the extraction site, primarily the resorption of about 50% of buccal bone width, but also a loss of buccal bone height.6 It is well known that these side effects can be minimized if implants are immediately placed with gap bone graft after extraction.
Implant stability is defined as having no clinical mobility, and the ability to support the axial, rotational, and lateral loads.7 It is important to check initial stability, since increased initial stability after placement affects long-term stability as well as the success rate of the implant. Subsequently, during osseointegration, bone is integrated near the implant thread, so that secondary stability is achieved by gradually increasing contact between bone and the implant.8 In particular, it is important to obtain initial and secondary stability when the implant is placed on a fresh extraction site with various bone defects.
Methods for measuring implant stability include histology, removal torque value (RTV) analysis, X-ray imaging, percussion testing, and magnetic resonance frequency analysis (RFA). However, most of these methods are not suitable for long-term clinical evaluation due to invasiveness or accuracy.9 RFA, a non-contact method, is a bending test of the bone-implant surface. A small magnetic bending force is applied to the side of a transducer mounted on the implant. The displacement, if any, of the implant is measured, confirming the clinical stability of the implant.8 With this device, the stability of the implant can be monitored at all stages of treatment.77 Predicting the osseointegration of implants or deciding load time using RFA value changes are considered positives in various studies. Of these advantages, RFA measurement is considered to be a relatively reliable implant stability measurement method.
Studies regarding RFA change in immediate placements are rare, and to our knowledge, no prior studies have tracked the stability of immediately placed implants with RFA. Performed retrospectively, the present study analyzed the RFA value changes of implants placed immediately after the extraction of the mandibular first molar, in order to evaluate changes in stability and to assist in deciding the load time. We hypothesized that the average RFA value at implant placement in the buccolingual and mesiodistal directions would be consistent.
Ⅱ. Materials and Methods
Patients who visited the Department of Oral and Maxillofacial Surgery at Inje University - Sanggye Paik Hospital from August 2012 to October 2019, and who underwent implant placement immediately after the extraction of a mandibular first molar, were selected for this retrospective study. The inclusion criteria were as follows:
1. RFA measurements were obtained during implant placement and impression-taking,
2. Surgery was performed without flap elevation,
3. A bone graft was placed between the implant and the extraction socket, and
4. Vertical loss of the buccal bone at the extraction socket was less than one-third
In total, 25 implants from 24 patients were included. Tooth extractions were performed to treat dental caries, tooth fractures, and endodontic or periodontal disease. Of the 24 patients, 12 were male and 12 were female, and the average age was 55.58 years (age range: 32–78 years). The majority (n = 17) of the patients were generally healthy, while 6 patients had systemic diseases such as diabetes mellitus (DM), hypertension (HTN), and myocardial infarction (MI), and 1 patient was breastfeeding. From the patients’ clinical records, the following types of information were obtained: sex, age, direction of implant, diameter and length of implant, RFA value during implant placement, and dental impressions (Table 1).
Table 1.
Demographic data of all patients
Preparation and surgery were performed in accordance with the general protocols as follows: 30 minutes prior to surgery the patient received intravenous antibiotics, after which an inferior alveolar anesthetic nerve block (2% articaine with 1:100,000 epinephrine) was performed, and the tooth was carefully extracted. The socket was debrided thoroughly post-extraction, and if necessary, a root dissection was performed to decrease trauma to the extraction socket. A sandblasted and acid-etched (SLA) surface implant was placed in the alveolar septum of the extraction site. If the septum did not exist, the implant was placed in the center of the extraction site. The implant insertion torque was measured in 5 Ncm units, and the insertion was finished in a non-submerged fashion for all implants. The gap between the implant and extraction socket was filled with bone graft material. If necessary, sutures were used to stabilize the peri-implant soft tissue. Postoperative medication was administered, including antibiotics and analgesics. Incision and flap elevations were not performed.
Using the Osstell ISQ scale (Osstell AB, Gothenburg, Sweden), the RFA value was measured during the implant placement and impression-taking, measuring the buccal, lingual, mesial, and distal sides. The RFA values were defined as follows, according to the manufacturer’s instructions: average RFA value in all directions (RFA1total), average RFA value in the buccolingual direction (RFA1BL), and average RFA value in the mesiodistal direction (RFA1MD) during implant placement; average RFA value in all directions (RFA2total), average RFA value in the buccolingual direction (RFA2BL), and average RFA value in the mesiodistal direction (RFA2MD) during impression-taking. The average time from implant placement to impression-taking was 1.82 months (range: 1-4 months). The changes in the RFA values between implant placement and impression-taking were verified using the Wilcoxon signed-rank test. SPSS Statistics 25.0 (IBM, USA) was used for statistical analysis.
Ⅲ. Results
Of the 25 implants, 16 replaced the right mandibular first molar, and 9 replaced the left. All implants in all patients healed uneventfully. The average diameter of the implants was 4.996 mm (range: 4.5-5.5mm), and the average length of the fixtures was 9.72 mm (range: 7.0-11.5 mm), as seen in Table 1. All implants were placed via flapless surgery, and soft tissue healing was achieved within 1-2 weeks. The average follow-up period was 24.2 months, during which none of the implants failed. During the impression-taking, all implants except one had RFA values higher than 65 in all directions: buccal, lingual, mesial, and distal (Table 1).
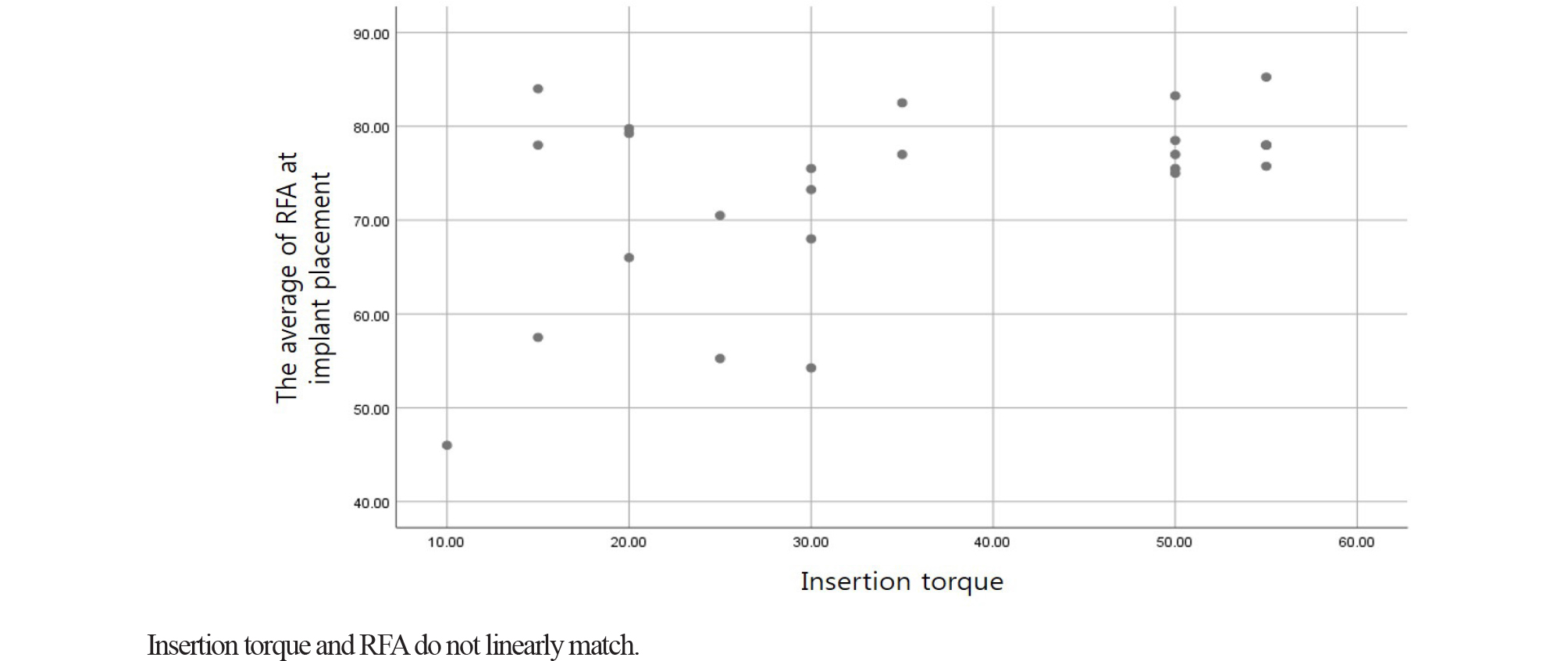
The average insertion torque was 35.2 Ncm (range: 10-55 Ncm). Spearman’s correlation analysis was used to examine the relationship between insertion torque and RFA value during implant placement. This analysis showed a low positive correlation, with a correlation coefficient = 0.335 (Table 2 and Fig. 1).
Table 2.
Spearman Correlation Coefficient Between RFA1total and Insertion torque value(RFA1total)
| Insertion torque | Spearman correlation coefficient | .335* |
| Significance | .102 | |
| N | 25 |
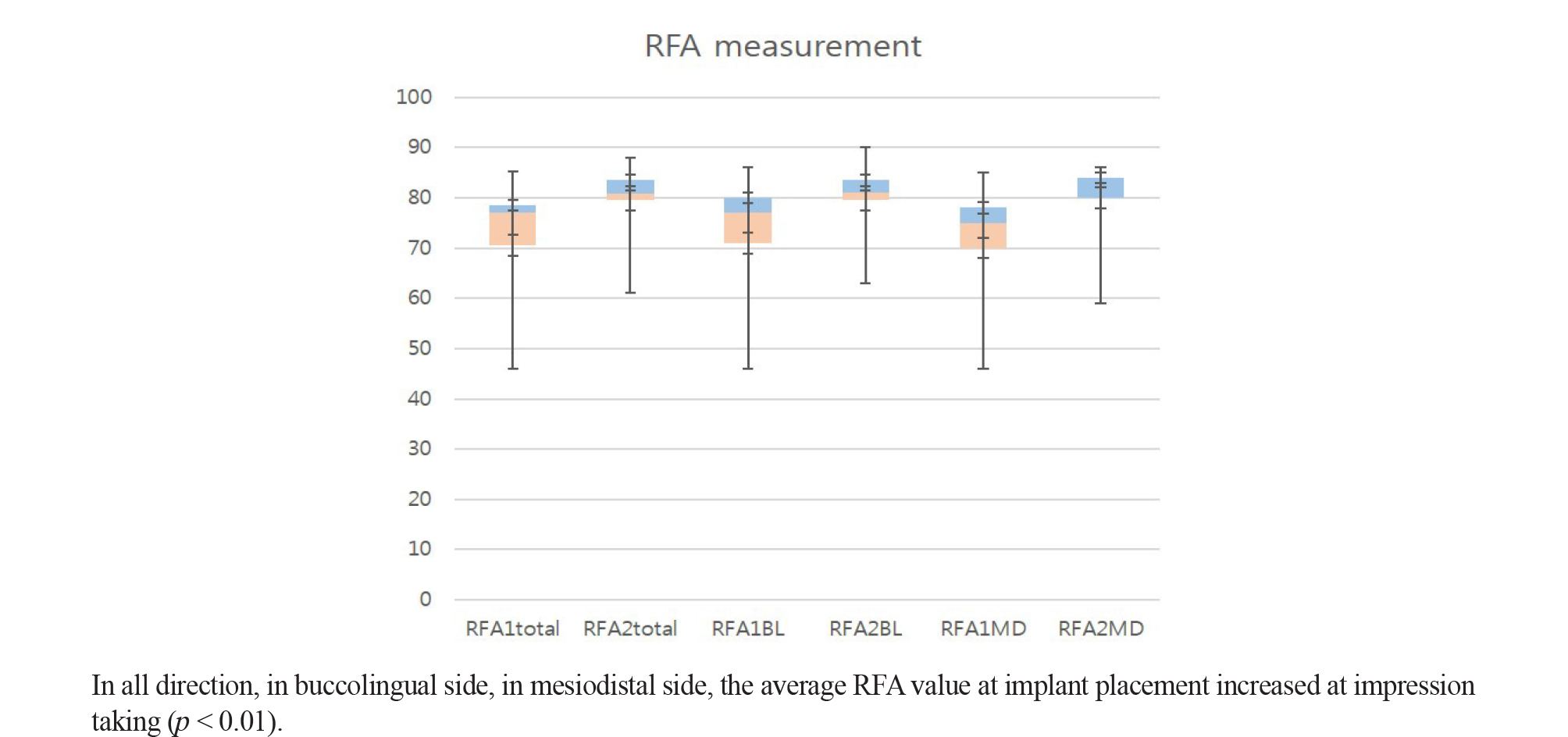
The average RFA values during implant placement were: all directions (RFA1total) = 73.24, buccolingual direction (RFA1BL) = 74.2, and mesiodistal direction (RFA1MD) = 72.28 (Table 3). The average RFA values during impression-taking were: all directions (RFA2total) = 79.64, buccolingual direction (RFA2BL) = 80.1, and mesiodistal direction (RFA2MD) = 79.18 (Table 3). The average RFA values for all directions, the buccolingual direction, and the mesiodistal direction, were increased, with a p-value < 0.01 (Fig. 2).
Ⅳ. Discussion
The method placing the implant immediately after extraction reduces the number of surgeries and procedures a patient undergoes, maintains alveolar bone height and width, and shortens the duration of the treatment period.10 Particularly in the placement of a single anterior implant, the immediate placement method is preferred because it maintains the shape of the buccal bone and soft tissue, and is a single-day restoration process, which does not impair aesthetics.11 Even with immediate placement, horizontal and vertical loss of the alveolar ridge cannot be avoided, although there is no visible bone loss affecting the initial stability.12 The immediate placement of a single implant in the posterior dentition is more difficult and less useful than for the anterior dentition, however, it can still reduce the duration of the treatment period and may prevent various difficulties and side effects that can occur during delayed placement.
During implant placement, the least invasive technique should generally be used. Although case selection is very important and technique sensitive,13 some authors are of the opinion that it is more important to place the implant with as little flap elevation as possible in order to minimize the loss of soft and hard tissue at the site of implant.14 Additionally, depending on the number and anatomy of the root and the presence of a septum, the difficulty and consideration for implant placement may change.15 There is a controversy regarding whether bone grafts and/or membranes are needed to fill various gaps between the implant and bone. Schwartz-Arad et al.16 reported that the survival rate of implants placed immediately after extraction is 93.9-100%, and if placed 3 to 5 mm below the root apex for initial fixation, gap-filling is not necessary and bone loss is not reduced by using a membrane. Tarnow et al.17 reported that if buccal bone is intact, gap-healing can be achieved sufficiently using only a membrane and stable initial sutures, without a bone graft. However, Chen et al.3, 18 have reported that when a rough surface implant is placed immediately after extraction, 2 mm or less of horizontal defect can be healed naturally without the use of a graft or a membrane, but that a horizontal defect of more than 2 mm or a damaged extraction socket requires the use both. In other literature, bone grafts and/or membranes can be applied to regenerate alveolar bone, providing a predictable and high-quality osseointegration.3
In practice, the primary stability of dental implants is one of the most important elements of successful osseointegration.19 Primary stability involving the mature bone around the extraction site plays a significant role in deciding the success and failure of an implant, especially in the immediate placement method.20 Primary stability can also be affected by factors such as bone quality and mass, surgical technique, implant design, and surface treatment.21 In general implant placement protocols, increased primary stability can be achieved by using a drill that is smaller than the diameter of the implant, or using a taper implant rather than a parallel implant in low-density bone with poor bone quality.22 If necessary, these protocols can be used for immediate implant placement to achieve better initial stability.
During implant placement surgery, stability can be measured by manually assessing immobility with the hand or by checking the insertion torque. Given the limitations caused by the invasiveness of the placement, it is common to evaluate immobility using a percussion test or magnetic RFA. Of these options, RFA evaluated immobility using a non-contact method, and therefore has the advantage of being noninvasive. Given this advantage, RFA is the more widely accepted method used to assess immobility. An RFA value that increases over time in an implant placed on a healed ridge can be considered to indicate an increasing stability of the implant, and may also indicate a successful osseointegration.23 As a result of osseointegration, mechanical stability then becomes biological stability, which can be determined by using RFA to evaluate the change in implant stability during osseointegration after implant placement.
Currently, there is no consensus on the minimum insertion torque or RFA value which defines a successful implant, 1 but it is known that there is a close relationship between the success of an implant and RFA value, as well as between success of implant and insertion torque.24 Rowan et al.25 has found that during implant placement, the average RFA value of implants placed immediately at various sites is lower than the average RFA values of delayed implants. At the postoperative check, however, after successful osseointegration, it has been confirmed that the average RFA values of immediately placed implants were consistently higher than 65, which was the minimum RFA value indicative of a successful implant. Balleri et al.26 have found that the RFA values of implants which have successfully undergone osseointegration was 57 to 82, and the average RFA value 1 year after implantation was 69. In other studies, an RFA value above 68 can be considered to indicate a high primary stability.27 Another previous study has showed that a successful implant has an average insertion torque of 33 Ncm and an average RFA value of 66.28 Becker et al.29 describes that implants with a low initial stability, determined by RFA measurement after immediate placement, tend to have an initial stability that increases over time, whereas implants with higher RFA values immediately after placement tend to have an initial stability that decreases over time. This suggests that all implants, regardless of the initial RFA value, reach a similar level of stability over time.
The initial stability of a failed implant decreases over time, until it is eventually lost.30 Nedir31 describes a proposed cutoff RFA value of 47, which indicates the failure of an implant. An RFA value of 47 or higher, therefore, should be considered to indicate a stable implant. According to another study, for 20 of 300 implants that failed, the average insertion torque was 21 Ncm and the average RFA value was 46.24 Repeated RFA measurements at certain intervals after the placement of an implant can assist in determining the load time27 and predicting early signs of clinical failure.32 Removing and connecting the transducer to the implanted fixture to take frequent RFA measurements can actually affect the healing of the implant. Yang et al.33 , however, reported no significant association between the removal or connection of the implant abutment and marginal bone resorption, implying that stability is not affected. From this, it may be inferred that the repeated removal and/or connection of the transducer does not affect the stability of the implant. Glauser et al.33 noted that 1-2 months after implant placement, the RFA value temporarily decreased to approximately 60. This initial decrease was due to relaxation over time of the compressed bone which resulted from the implant placement. The initial decrease is associated with biological changes and marginal bone resorption which are part of the early healing process. After a 12 month implant stabilization period, the RFA value started to increase again,27, 33 primarily the result of increased stiffness caused by osseointegration and bone maturation at the implant site.31
In the present study, the implant was placed immediately after the extraction of the mandibular first molars with flapless surgery and a gap bone graft. The insertion torque varied from 10 to 55 Ncm, as seen in Table 1. Even in the sixth patient, who had the lowest insertion torque, an increased RFA value over 10 was observed in all directions after 2.5 months, at the time of impression-taking, after which stable loading was performed. At the time of the implant placement, the RFA value was over the cutoff value of 47 in all directions (Nedir et al. 2004) for all patients except for one, which indicated implant failure in that patient. At the time of impression-taking, RFA values of 65 or more were observed in all directions in all patients except for one, whose RFA value was 47 or more in all directions. Comparing the average RFA values in all directions during implant placement (RFA1total) to those at the time of impression-taking (RFA2total), it was confirmed that the average RFA value in all directions had increased. Comparing the average RFA values in the buccolingual direction during implant placement (RFA1BL) to those at the time of impression-taking (RFA2BL), it was confirmed that the average RFA value in the buccolingual direction had increased. Comparing the average RFA values in the mesiodistal direction during implant placement (RFA1BL) to those at the time of impression-taking (RFA2BL), it was confirmed that the average RFA value had also increased in the mesiodistal direction. After immediate placement, osseointegration and socket healing were achieved, and subsequently, secondary stability of the implant was obtained. The results of the present study confirmed that the stability of the implant increased as the RFA values increased, regardless of the direction.
The RFA values in the buccolingual and mesiodistal directions during implant placement showed statistically significant differences. It is thought that this is due to the bone-implant contact pattern, which can be formed in a variety of manners as the result of the different extraction socket shapes found during immediate implant placement. A higher RFA value was observed in the buccolingual direction than in the mesiodistal direction, which is presumed to be due to the anatomical shape of the region. When an implant is placed in the mandibular first molar region, in the mesiodistal direction there is only a small apical area where alveolar bone is in contact with the implant, but in the buccolingual direction, the implant is placed closely to lingual bone in the extraction socket, creating a wider bone-implant surface. Additionally, the alveolar septum at the extraction site, which can offer primary stability, is often present in the buccolingual direction. This allows for the inference that since bone-implant contact, which affects stability, occurs more in the buccolingual direction, the RFA value would indicate an effect on stability.
Table 2 shows a slight non-linear relationship between insertion torque and RFA value, (Spearman correlation coefficient = 0.335), which is similar to a previous study which indicated that insertion torque and RFA did not have a high correlation.22 It seems that in an immediate placement, due to bone defect that remains after the extraction, the bone-implant contact may form differently in the buccolingual and mesiodistal directions, and that the bone-implant contact required for stability may be limited to the root area. RFA and insertion torque represent resistance to two different characteristics of primary stability. RFA represents resistance to bending load, and insertion torque represents resistance to shear force.34 These representations are related to the above in terms of stability. Given that the RFA value does not linearly correspond with implant stability, it can be inferred that the relationship between insertion torque and stability as measured by RFA is not completely linear (Fig. 1). Scarano et al.35 have found a statistically significant correlation between RFA value and bone-implant contact. In other studies, RFA is related to the height of the portion of the implant which is not surrounded by bone and the stability of the bone-implant surface. Rigidity of the bone-implant surface, bone quality, and the rigidity of the implant component itself all affect the RFA value.36 It is thought that the results described above are caused by the differences in bone-implant contact, which can occur during implant placement, and the different principles of each measurement method.
In the present study, there was a delay 1-4 months, with an average time of 1.82 months, between the implant placement and the impression-taking. During of the average 24.2 months of follow-up, there were no implant failures. The authors have therefore tentatively determined the timing of impression-taking in consideration to the RFA value, insertion torque, and bone-implant contact at placement. The RFA value was measured on the date scheduled for impression-taking, and the decision of whether or not to proceed with the next step was made. Osseointegration of the SLA implant surface with healthy bone was complete after approximately 6-8 weeks. With the immediate placement method, it may take longer for bone formation and osseointegration to occur in the gap of the coronal area. However, if the RFA value is increased, osseointegration is expected to proceed well, particularly where the bone-implant contact is intact or the gap is narrow from the time of placement, such as in the apical area. Therefore, at that time it is appropriate to proceed with the restoration stage for implant loading.
Some patients had systemic diseases, such as DM, HTN, and MI. In general, the primary stability, as indicated by the RFA value, is low in diabetic patients, and if not controlled, the RFA value after 1 year is even lower than that of controlled patients.37 Therefore, an individualized plan systemic disease control must be completed prior to implant placement.38 In the present study, the initial RFA values and subsequent RFA value increases in patients with systemic diseases were comparable to those in patients without systemic disease. Although the present study only included small number of patients, it can still be expected that if a systemic disease is well controlled in patients who undergo an immediate implant placement after extraction, the disease is unlikely to affect the primary and secondary stability of the implant.
The biggest limitation to the present study was the small number of patients included in the study population. The differing lengths and widths of the implants make it rather difficult to confirm an association with RFA values in this regard. There is also the limitation due to the inconsistent time between the implant procedure and the impression-taking. Another limitation is that the marginal bone level changes were not measured, and it is thought to be helpful to confirm long-term success by measuring these changes. In addition, a few cases with short follow-up periods have been included, and it is therefore difficult to confirm the long-term stability and prognosis of implants in some of these cases. Under limited conditions, the RFA values tended to increase with the immediate placement of the implant after the extraction of the mandibular first molar and showed stable clinical results. Compared to the delayed placement method, the bone gap at the extraction site and the irregularity of bone-implant contact are potential complications with the immediate placement method. Whether bone grafting is performed or not, how much these bone gaps affect the osseointegration and healing period of implants, and when to apply loading remain concerns for many clinicians when assessing immediate versus delayed placement. The results of the present study indicate that the RFA values of implants placed immediately after the extraction of the mandibular first molar tend to increase, as is seen with the delayed placement method.
Ⅴ. Conclusion
Although the amount of data is limited in the study, an RFA value which increases over time could be used as an indicator to help evaluate the healing of the implant and to determine the load time after the immediate placement of the implant after the extraction of mandibular first molar. Further studies with larger study populations are needed in the future.